This new study reveals venetoclax and rituximab effectively treat chronic lymphocytic leukemia, showing high response rates and prolonged progression-free survival.

This new study reveals venetoclax and rituximab effectively treat chronic lymphocytic leukemia, showing high response rates and prolonged progression-free survival.

A Maui Derm 2026 session explored advancements in pediatric dermatology, emphasizing precision medicine and targeted therapies for children.

Participants in the pilot program had high baseline knowledge about their chronic spontaneous urticaria, but the same was not always true of their physicians.

Speakers delved into innovative strategies in skin tumor management, focusing on chemotherapies, patient risk assessment, and evolving treatment protocols.
The upcoming price negotiations are the third cycle since the Inflation Reduction Act was passed in 2022.

LLMs provide valuable answers to common Mohs surgery questions, supporting postoperative care and patient education without replacing physician guidance.

AAP’s 2026 immunization schedule continues protection against 18 diseases as the CDC reduces its recommendations to 11.

Experts discuss groundbreaking therapies in dermatology at Maui Derm 2026, addressing complex skin diseases and enhancing patient care with innovative treatments.
Experts raise concerns that CDC childhood immunization changes could reduce vaccine uptake and weaken trust in public health guidance.

Nate C. Apathy, PhD, guest editor of AJMC's 15th Health IT special issue, calls for tools to be closely aligned with user needs.

Minnesota physicians warn ICE activity in hospitals and clinics is deterring care, undermining patient safety, and worsening health outcomes.

CMS’s 2027 proposed MA payment rate increase of .09% falls short of expectations, potentially increasing premiums and reducing benefits for seniors.
With the CDC’s decision to roll back recommendations to vaccinate for several conditions, questions have arisen as to what that may mean in the US.

Daniel Siegel, MD, discusses a loophole he looks out for in ICD-10 coding and billing.

Almost half of the monthly-updated CDC databases had unexplained pauses in 2025, mostly affecting vaccination data, raising transparency and public health concerns.
ACE inhibitors may improve survival in IPF patients, highlighting potential repurposing of a common cardiovascular drug.

Maui Derm 2026 showcases groundbreaking clinical trial data and innovative strategies in dermatology.

Experts underscored that educating clinicians and the public about red flag CRC symptoms could facilitate earlier screening, diagnosis, and treatment.

Explore how network integrity enhances managed care strategies, driving value-based care success and improving patient outcomes in health systems.

Adults who are underweight, overweight, or obese face higher medical expenditures, varying by age, sex, and care setting.

Ongoing phase 3 trials target improved therapies for hematologic malignancies, focusing on CLL, multiple myeloma, and pediatric leukemia outcomes.
These findings support the potential role of ctDNA in prognostic assessment and early intervention.
A review article focused on first-line treatment of chronic lymphocytic leukemia highlights the importance of shared decision-making.

Patients with allergic rhinitis face prolonged symptoms and inadequate treatment, highlighting a need for better awareness and access to allergen immunotherapy.
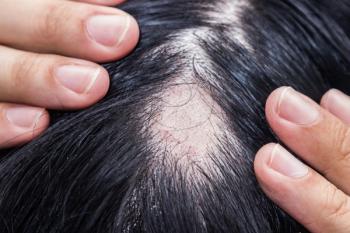
Central centrifugal cicatricial alopecia (CCCA) faces significant diagnostic delays, highlighting the need for increased awareness and education among providers.

Research reveals that socioeconomic status significantly impacts survival rates in young adults with metastatic colorectal cancer, emphasizing the need for health equity initiatives.
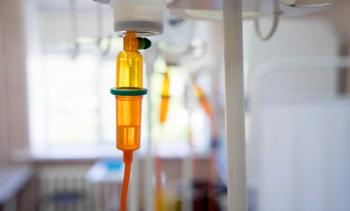
Phase 2 trial results show anlotinib with anthracyclines and ifosfamide yields a 30.8% ORR and an 82.7% DCR in advanced soft tissue sarcoma.

Health outcomes and inpatient costs improved for veterans when the federal government invested in preventing homelessness.

A randomized clinical trial found a voice-enabled virtual assistant reduced mental distress and improved quality of life, self-care, and glycemic control.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
