Geisinger Health Plan is waiving coronavirus disease 2019 (COVID-19) testing- and treatment-related costs; President Donald Trump comes down hard on the World Health Organization; there is optimism that schools could reopen in the fall.

Geisinger Health Plan is waiving coronavirus disease 2019 (COVID-19) testing- and treatment-related costs; President Donald Trump comes down hard on the World Health Organization; there is optimism that schools could reopen in the fall.

Almost 70% of the world’s population could be living in urban areas, being continuously exposed to air pollution, by 2050, while cases of dementia are expected to triple. Recent study results highlight the link between cardiovascular disease and dementia, as mediated by long-term exposure to air pollution.

Women who experience migraine with aura have an increased hazard of cardiovascular disease–specific mortality compared with individuals with no history of headache, according to a study published in The Journal of Headache and Pain.
Anterior and posterior ocular structure changes occur in astronauts who complete long-term spaceflights, according to a study published in JAMA Ophthalmology.

Ashwagandha root extract, a revered herb of Ayurveda, was associated with improvements in quality of life, sleep quality, and mental alertness in elderly patients, according to study findings.

Researchers created a machine-learning–based model to help predict which patients will develop diabetes, according to an abstract to be published in the Journal of the Endocrine Society.

The rate increase comes as the agency is easing up on quality reporting requirements to give health sytems breathing room amid the pandemic. Monday’s announcement also clarified some payment changes for end-stage renal disease.

Patients with cancer are reporting that they have faced treatment delays due to the novel coronavirus disease 2019 (COVID-19); the Trump administration reached an agreement with 3M Co. to produce nearly 167 million face masks in the next 3 months; US hospitals cite lack of supplies, testing coordination as major obstacles during the pandemic, according to an HHS survey.

There is a great need for additional outreach to the transgender community, especially by addressing concerns that include fear and loss of confidentiality, distrust of the medical community, abandonment, and abuse.

Cushing syndrome, a rare endocrine disorder caused by abnormally excessive amounts of the hormone cortisol, has a new pharmaceutical treatment to treat cortisol overproduction.

Repetitive transcranial magnetic stimulation of the brain has been shown to reduce the effects of treatment-resistant depression, but study results show that intermittent theta-burst stimulation may be more efficient and effective.
Parkinson disease (PD) subtypes derived from a novel subtyping system were significantly linked with disease duration and severity. However, the system may solely reflect stages of PD, rather than identify distinct clinical subtypes.
British Prime Minister Boris Johnson was moved to the intensive care unit (ICU) of a London hospital, 10 days after being diagnosed with coronavirus disease 2019 (COVID-19). Also Monday, the CDC released a preliminary report about how COVID-19 is affecting children in the United States.
In a study released Monday by the Integrated Benefits Institute, researchers found that due to lost time from work caused by the novel coronavirus disease 2019 (COVID-19), employee benefits for absent workers could cost employers more than $23 billion.

As the United States prepares for what could be the worst week yet of the coronavirus disease 2019 (COVID-19) pandemic, national attention has been focused on the disease’s current epicenters in New York and New Jersey. However, across the country, the pandemic is slowly seeping into the nation’s economically vulnerable populations and is already taking a toll on minority communities.
Kimberly Lovett Rockwell, MD, JD, and Alexis S. Gilroy, JD, the authors of a commentary in the April issue of The American Journal of Managed Care®, explain how telemedicine can help alleviate the burden on health systems brought by the coronavirus disease 2019 (COVID-19) pandemic and how regulations are shifting to enable use of the technology during the pandemic and beyond.

Coronavirus disease 2019 (COVID-19) was linked with cardiovascaluar issues and cardiac arrest; African Americans were shown to have higher rates of infection and death due to COVID-19; health experts call for smokers to cease use and manufacturers to halt production.

Welcome to Paper of the Week, which looks back at research and commentary of the past 25 years in The American Journal of Managed Care® and why it matters today.

The analysis looked at 13 of America’s largest employers, including energy, hospitality, retail, transportation, and finance companies.

The FDA has announced a relaxing of its restrictions on gay men being allowed to donate blood, in light of the coronavirus disease 2019 pandemic. Instead of 1 year, if a male has had sex with another male, he need only wait 3 months to donate blood.

What can be done to further delineate the risk factors associated with breast cancer to increase prevention efforts across the board? The key may lie in the white blood cells that circulate in the blood, particularly leukocytes and monocytes.

The introduction of biosimilars in the US market brings along specific challenges to health system pharmacists, including formulary assessment, implementation, and education of patients and various health system stakeholders, according to a review published in Drugs In Context.

This week, the top managed care news includes a CDC report says diabetes is the condition most linked with coronavirus disease 2019, CMS temporarily suspends rules to give hospitals more capacity during the COVID-19 pandemic, and a late-stage trial for dapagliflozin ends early after showing efficacy for chronic kidney disease.

Detectable viral load (VL) is associated with a significantly increased risk of cardiovascular disease (CVD) among US youth living with HIV, according to an abstract presented at the Conference on Retroviruses and Opportunistic Infections.

Coverage of our peer-reviewed research and news reporting in the healthcare and mainstream press.

Drugs used by hospitals to intubate and treat patients with COVID-19 are in short supply; rapid tests will be sent to Native American, rural populations; data shows a rise in nonfatal drug overdoses between 2016 and 2017.

Mantle cell lymphoma is a type of B-cell non-Hodgkin lymphoma with a typically poor prognosis. Even with an allogeneic stem cell transplant, patients can become resistant to chemotherapy. Most do not survive 4 or 5 years after diagnosis, and the 10-year survival rate hovers between 5% and 10%.
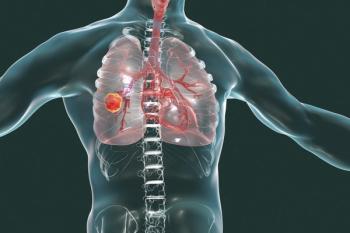
A large-scale study reports that patients with chronic obstructive pulmonary disease (COPD)—even if they never smoked—have a 2.6 times greater incidence of developing lung cancer compared with those who had neither COPD or a history of smoking.

In a recently published review, researchers examined the brain-skin connection and the pathogenesis of psoriasis, focusing on the serotonergic system.

Theranexus announced this week that its phase 2 trial for THN102 (modafinil/flecainide) in patients with Parkinson disease met its primary efficacy end point of significantly increasing the proportion of patients no longer suffering from excessive daytime sleepiness.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
