
Myasthenia Gravis
Latest News
Latest Videos

CME Content
More News

Patients with myasthenia gravis experience significantly higher rates of urinary incontinence and overactive bladder symptoms, impacting their quality of life.

Jonathan Strober, MD, discusses promising results from the VIBRANCE-MG trial, highlighting nipocalimab's safety and efficacy for pediatric myasthenia gravis.

Patients with generalized myasthenia gravis face significant barriers to diagnosis, creating the need for improved patient–health care interactions.

Discover the latest breakthroughs in myasthenia gravis treatment, including rozanolixizumab and nipocalimab, and insights on disease burden and dysphagia indicators.

Inebilizumab gains FDA approval for treating generalized myasthenia gravis, showing significant improvements in muscle strength and steroid reduction.

Minimally invasive thymectomy for ocular myasthenia gravis (MG) enhanced complete stable remission rates and reduced surgical risks.

KYV-101 shows promising efficacy in treating generalized myasthenia gravis, achieving significant symptom reduction and demonstrating a novel CAR T-cell approach.

Rozanolixizumab shows promise in treating triple-seronegative myasthenia gravis, offering hope for patients with limited treatment options.

Gefurulimab shows significant improvements in myasthenia gravis (MG) symptoms, offering a convenient self-administered treatment option for patients.

Remibrutinib shows promise in treating generalized myasthenia gravis, with ongoing trials assessing its efficacy and safety for patients.

Experts discuss personalized myasthenia gravis treatment, emphasizing patient education and engagement for optimal care and advocacy in managing this variable disease.

Ratna Kiran Bhavaraju-Sanka, MD, and Beth Stein, MD, advocate for improved proactive management of myasthenia gravis (MG) for better patient outcomes.

Thorough eye exams in patients who have myasthenia gravis can detect concurrent autoimmune retinopathy and its complications.

Patients successfully self-administer rozanolixizumab for generalized myasthenia gravis (gmG), preferring manual push delivery over infusion pumps in a phase 3 trial.

Efgartigimod shows promising efficacy over IV immunoglobulin in treating impending myasthenic crisis in patients with myasthenia gravis.

Patients with myasthenia gravis had more COVID-19–related anxieties due to their diagnosis and immunosuppressive therapies.

Efgartigimod shows promising early response in generalized myasthenia gravis, especially in patients with short disease duration and severe bulbar symptoms.

Long-term follow-up data on the use of BCMA-directed RNA chimeric antigen receptor T-cell therapy (CAR T) for refractory generalized myasthenia gravis show patient outcomes at 2, 3, 6, 9, and 12 months.

Efgartigimod shows promise as a groundbreaking treatment for seronegative generalized myasthenia gravis (gMG), addressing a critical unmet need in patient care.

Using US claims data, the authors evaluated oral glucocorticoid (GC) use at 5 time points during their retrospective analysis: 3 months before starting efgartigimod and 3, 6, 9, and 12 months after starting efgartigimod.

The humanized IgG4 monoclonal antibody was first approved for myasthenia gravis in the US in 2023, with approvals in Japan and the European Union following later that year and 2025, respectively.
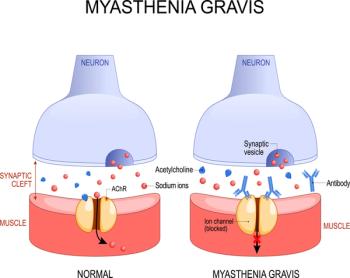
Despite nurses often being the first health care workers many patients encounter, they frequently lack adequate tools to optimally evaluate their patients’ conditions, as in the case with myasthenia gravis and being able to predict patients at risk of myasthenic crisis.

Among patients who have myasthenia gravis, even low disease activity can impact their quality of life (QOL) via motor fatiguability.

Fear of progression is common among patients living with chronic diseases, but the degree to which it interferes with patient outcomes, including treatment adherence, deserves further investigation, study authors note.

The primary end point in PREVAIL is Myasthenia Gravis-Activities of Daily Living total score improvement from baseline, and the secondary end points are Quantitative Myasthenia Gravis total score and Myasthenia Gravis Composite total score.












