
In a pair of lively debates at the International Stroke Conference 2026, experts from around the world discussed controversies in stroke care.

Rose is an editorial director at The American Journal of Managed Care® (AJMC®).
She has a BA in journalism & media studies and Spanish from Rutgers University. You can connect with Rose on LinkedIn.

In a pair of lively debates at the International Stroke Conference 2026, experts from around the world discussed controversies in stroke care.

OCEANIC-STROKE shows asundexian cuts recurrent ischemic stroke risk across noncardioembolic subtypes without increasing major bleeding risk.

Experts at ISC 2026 highlighted growing evidence that GLP-1 receptor agonists may reduce stroke risk and support brain health through anti-inflammatory, vascular, and neuroprotective mechanisms.

OCEANIC-STROKE shows asundexian cuts ischemic stroke recurrence by 26% in patients taking standard antiplatelet therapy, with no major bleeding increase.

Yearly dental visits were associated with fewer secondary strokes, and hyperthyroidism was flagged as a key CVST risk factor in women.

Clearer definitions, tailored programs and policies, and telestroke care could help close rural stroke care disparities.

CHOICE2 found intraarterial alteplase after thrombectomy raised the odds of excellent outcomes following large-vessel occlusion ischemic stroke.

Margaret Krackeler, MD, of Kaiser Permanente Northern California, discusses the growing role of real-world treatment data in CLL and other cancers.

Margaret Krackeler, MD, discusses real-world data supporting the switch from ibrutinib to zanubrutinib for chronic lymphocytic leukemia.
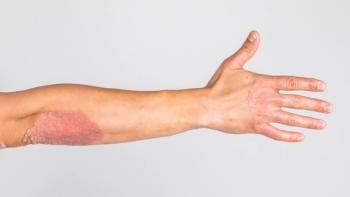
Tildrakizumab at 100-mg and 200-mg doses showed long-term effectiveness for managing psoriasis in challenging areas in a real-world study.

Vutrisiran significantly reduces mortality and cardiovascular events in older patients with ATTR-CM, enhancing quality of life and functional capacity.

Adopting zanubrutinib for the treatment of chronic lymphocytic leukemia showed improved tolerability and persistence, enhancing patient safety and outcomes.

Patients with allergic rhinitis face prolonged symptoms and inadequate treatment, highlighting a need for better awareness and access to allergen immunotherapy.
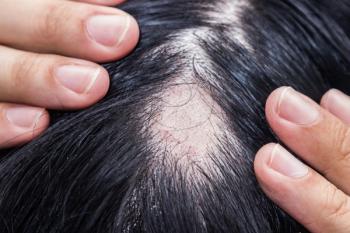
Central centrifugal cicatricial alopecia (CCCA) faces significant diagnostic delays, highlighting the need for increased awareness and education among providers.

There is a shift happening in mental health care, with real-world data and holistic evaluations improving patient outcomes and reducing disparities.

Trump's Great Healthcare Plan aims to lower drug prices, reduce insurance premiums, and enhance transparency, according to the White House.
Patients with mantle cell lymphoma experienced better survival rates when treated at academic centers compared with community facilities.

Scott Soefje, PharmD, outlines proactive financial screening, flexible payment models, and stronger academic-community partnerships for advanced cancer care.

The most-read articles focused on soft tissue sarcoma research, including innovative biomarkers, treatment strategies, and multidisciplinary care challenges.

The 2025 Community Oncology Conference, hosted by COA, reflected a commitment to patient-centered care, access, and innovation in community oncology.
In-home step training for multiple sclerosis showed no improvement in ankle proprioception or muscle performance, highlighting challenges in exercise adherence.

Explore the latest breakthroughs in allergy treatments, including FDA approvals and emerging therapies that enhance patient care and address systemic challenges.

Scott Soefje, PharmD, MBA, BCOP, of Mayo Clinic, discusses how payer-provider collaboration and pharmacist-led management can help improve oncology care.

The approval was based on the pivotal phase 3 FIBRONEER-ILD trial, in which nerandomilast slowed lung function decline in PPF and had similar discontinuation rates to placebo.

The 2025 American Thoracic Society International Conference brought advancements in pulmonary medicine and highlighted the need for health equity reforms.
Community oncology practices can enhance their presence in the community by offering innovative service lines such as multispecialty infusions, and by offering enrollment assistance for better outcomes.
Pirtobrutinib significantly improved progression-free survival in treatment-naïve CLL compared with bendamustine plus rituximab.

Academic and clinical experts convened in New Haven, Connecticut, on October 30, 2025, to discuss the potential of coordinated care and prevention to manage cardio-renal-metabolic (CRM) disease.

Ryan Haumschild, PharmD, discusses overcoming barriers to cancer care access, emphasizing the role of pharmacy in improving access and affordability.

New treatment options for idiopathic pulmonary fibrosis (IPF) and progressive pulmonary fibrosis (PPF) could enhance patient care and monitoring.

Published: April 7th 2025 | Updated:
Published: May 21st 2023 | Updated:

Published: December 24th 2020 | Updated:
Published: June 5th 2025 | Updated:

Published: September 5th 2025 | Updated:
Published: November 23rd 2021 | Updated:

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
