Feature
Article
FDA Approves Optune Lua Device in NSCLC
Author(s):
Key Takeaways
- Optune Lua is FDA approved for use with immunotherapy or docetaxel in metastatic stage IV NSCLC post-platinum treatment.
- The device uses tumor treating fields to disrupt cancer cell division, offering a nontoxic treatment option.
Optune Lua creates tumor-treating fields to disrupt cancer cell division, and it is used in conjunction with PD-1/PD-L1 inhibitors or docetaxel to treat metastatic non–small cell lung cancer (NSCLC) that has not responded to platinum-based treatment.
Optune Lua (Novocure) is now approved by the FDA for concurrent use with either immunotherapy (PD-1/PD-L1 inhibitors) or docetaxel, a taxane, for adults patients whose metastatic stage IV non–small cell lung cancer (NSCLC) has advanced despite platinum-based treatment.1
The wearable and portable device was first approved in 2019 under the Humanitarian Device Exemption pathway in combination with chemotherapy to treat malignant pleural mesothelioma,2 which had not had a treatment approved by the FDA in 15-plus years,3 and is a type of lung cancer that develops in the lining outside of the lungs and is typically attributed to asbestos exposure.4 The device is aIso approved in certain countries for use among adult patients who have glioblastoma and pleural mesothelioma.1
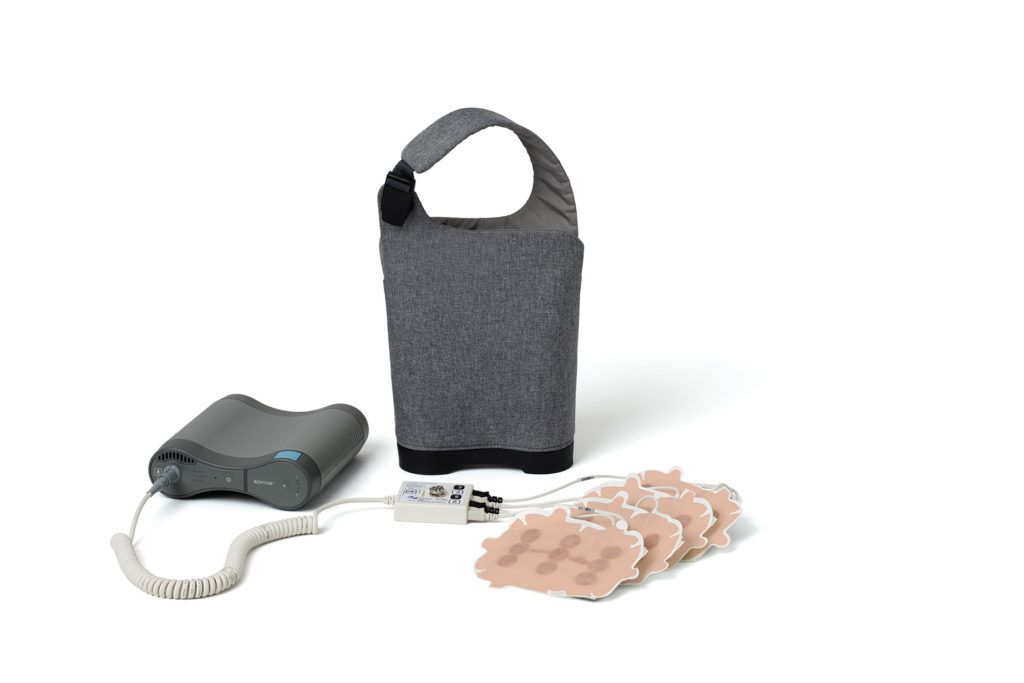
Optune Lua works through the use of 4 transducer arrays (adhesive patches) that produce tumor-treating fields (electric fields) that work to interrupt cancer cell development by slowing down or stopping cancer cell division.5
Optune Lua | Reproduced with permission from Novocure GmbH ©2022 Novocure GmbH – All rights reserved
This newest indication is backed by overall survival data from the prospective, randomized, open-label, multicenter phase 3 LUNAR trial (NCT02973789),1 which evaluated the device among patients with squamous or nonsquamous stage IV NSCLC, who were assigned in a 1:1 randomized fashion to docetaxel plus the NovoTTF-100L System (Optune Lua’s name while being investigated) or immune checkpoint inhibitor treatment plus the NovoTTF-100L System. Patients received continuous treatment with the system until disease progression according to Response Evaluation Criteria In Solid Tumors (RECIST) or immune-related RECIST, per treatment with docetaxel or an immune checkpoint inhibitor, respectively.6
“There have been a number of important advances in first-line treatment for NSCLC, but this is an aggressive disease, and most patients will develop progression, with limited effective treatment options in second line and beyond,” Ticiana Leal, MD, associate professor and director of the Thoracic Oncology Program at the Winship Cancer Institute of Emory University School of Medicine and primary investigator of the LUNAR study, said in a statement. “The overall survival results we observed with Optune Lua in the LUNAR study mark the first substantial improvement in more than 8 years in this patient population which, when combined with Optune Lua’s lack of systemic toxicity, make this a compelling development for many patients and their physicians who need better treatment options for this advanced disease.”
In the LUNAR trial, the experimental arm saw 145 patients receive treatment with Optune Lua with immunotherapy or docetaxel, and the control arm had 146 patients receive treatment with PD-1/PD-L1 inhibitors or docetaxel alone. The primary end point was statistically significant and clinically meaningful OS, which was 3.3 months better (P = .04) in the experimental study cohort vs the control group. The median OS was 13.2 (95% CI, 10.3-15.5) months vs 9.9 (95% CI, 8.2-12.2) months, respectively.1
There were also 2 secondary end points. Median OS from Optune Lua plus a PD-1/PD-L1 inhibitor (n = 70) compared with just a PD-1/PD-L1 inhibitor (n = 71) was 19.0 (95% CI, 10.6-28.2) months vs 10.8 (95% CI, 8.3-17.6) months, respectively, and considered statistically significant (P = .02). Median OS from Optune Lua plus docetaxel (n = 75) compared with docetaxel alone (n = 75) was 11.1 (95% CI, 8.2-13.9) months vs 8.9 (95% CI, 6.5-11.3) months, which trended positive but was not considered statistically significant.
Two-thirds of patients experienced dermatologic device-related adverse events (AEs), manifested as skin irritation from the adhesive patches, but most of these AEs were grade 1; there were only 6 reports of grade 3 skin toxicity, which required treatment interruption; no grade 4/5 AEs; and no device-related deaths. The most common AEs include alopecia, anorexia, localized edema (swelling), skin ulcers, hypokalemia (low potassium levels), dysphagia, and pain in the muscles, bones, or joints.
The median patient age was 66 (range, 22-86) years, 34% were female patients, and 96% had an ECOG performance status of 0 to 1, indicating they had no or only slight restrictions on their physical capabilities.7
Contraindications for use of Optune Lua are having an electrical implant and known sensitivity to gels used for ECG stickers of transcutaneous electrical nerve stimulation electrodes. The device, which cannot get wet, is also not recommended for women who are pregnant or planning to attempt pregnancy, and should only be used after having been trained on its use.1
References
1. FDA approves Novocure’s Optune Lua for the treatment of metastatic non-small cell lung cancer. News release. Novocure. October 15, 2024. Accessed October 16, 2024. https://www.novocure.com/fda-approves-novocures-optune-lua-for-the-treatment-of-metastatic-non-small-cell-lung-cancer/
2. How is malignant pleural mesothelioma treated? Novocure. August 2024. Accessed October 16, 2024. https://www.optunelua.com/about-mesothelioma/treating-mpm#mesothelioma-treated
3. Optune Lua. Novocure. August 2024. Accessed October 16, 2024. https://www.optunelua.com/
4. Moncivais K. Mesothelioma vs. lung cancer. Mesothelioma.com. Accessed October 16, 2024. https://www.mesothelioma.com/asbestos-cancer/mesothelioma-vs-lung-cancer/#:~:text=Is%20mesothelioma%20the%20same%20as%20non%2Dsmall%20cell%20lung%20cancer,that%20develops%20inside%20the%20lungs
5. How Optune Lua works. Novocure. Accessed October 16, 2024. https://www.optunelua.com/about-optune-lua/how-optune-lua-works
6. Effect of tumor treating fields (TTFields) (150 kHz) concurrent with standard of care therapies for treatment of stage 4 non-small cell lung cancer (NSCLC) following platinum failure (LUNAR). ClinicalTrials.gov. Updated September 26, 2024. Accessed October 16, 2024. https://clinicaltrials.gov/study/NCT02973789
7. ECOG performance status scale. ECOG-ACRIN Cancer Research Group. Accessed October 16, 2024. https://ecog-acrin.org/resources/ecog-performance-status/
Newsletter
Stay ahead of policy, cost, and value—subscribe to AJMC for expert insights at the intersection of clinical care and health economics.