There has not been a change in the standard of care in this type of cancer in a decade, according to an expert from ASCO.
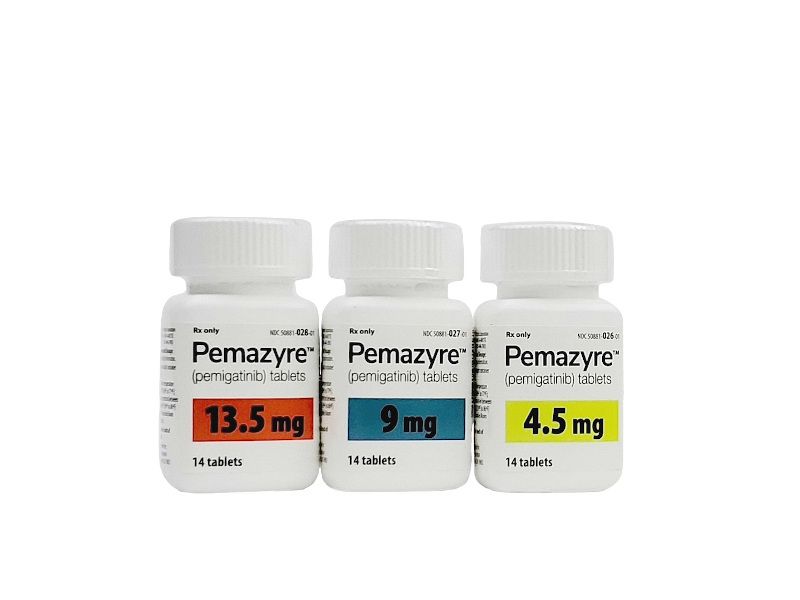
Drugs targeting FGFR mutations, which are seen in patients with intrahepatic cholangiocarcinoma (CCA), have generated particular excitement; the authors note that prior to the approval of pemigatinib, the first targeted therapy approved by FDA, only 15% to 25% of patients with CCA were “fit enough to receive second-line chemotherapy.”
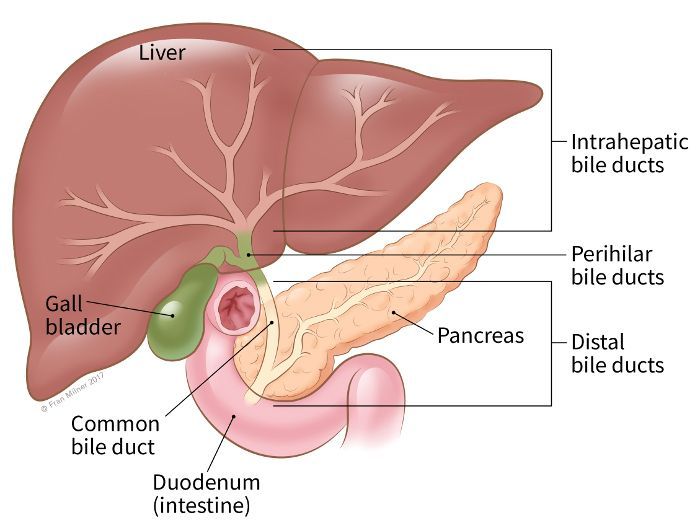
What Makes Recurrence More Likely After Hepatectomy in Intrahepatic Cholangiocarcinoma?
What Do Real-world Data Say About FGFR2 Status and PFS, OS in Cholangiocarcinoma?

There has not been a change in the standard of care in this type of cancer in a decade, according to an expert from ASCO.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
