Taletrectinib was added to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology as a preferred option for the first-line and subsequent treatment of advanced ROS1-positive non–small cell lung cancer (NSCLC).

Taletrectinib was added to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology as a preferred option for the first-line and subsequent treatment of advanced ROS1-positive non–small cell lung cancer (NSCLC).

This next-generation ROS1 tyrosine kinase inhibitor previously received breakthrough therapy and orphan drug designations for the same patient population, as well as additional non–small cell lung cancer (NSCLC) indications.
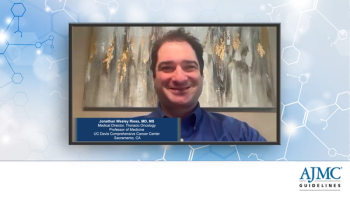
An expert discusses key challenges in advancing care for ROS1-positive non-small cell lung cancer, including the rarity of the disease and difficulty enrolling patients in clinical trials, while highlighting the importance of access to next-generation therapies, the impact of updated 2025 NCCN guidelines recommending taletrectinib, and the critical need for timely, comprehensive molecular testing to guide targeted treatment and avoid ineffective immunotherapy.

An expert discusses the personalized approach to treating ROS1-positive non-small cell lung cancer, emphasizing the preference for next-generation TKIs like taletrectinib and repotrectinib due to their CNS activity and efficacy against resistance mutations, particularly G2032R, while highlighting the importance of molecular profiling, avoiding immunotherapy, and considering clinical trials or chemotherapy in later-line settings.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
