
Medicare patients can now get the FreeStyle Libre 2 continuous glucose monitor (CGM); Anthony S. Fauci, MD, discussed the chance of an early release of a COVID-19 vaccine; US backs out of World Health Organization’s vaccine initiative.

Medicare patients can now get the FreeStyle Libre 2 continuous glucose monitor (CGM); Anthony S. Fauci, MD, discussed the chance of an early release of a COVID-19 vaccine; US backs out of World Health Organization’s vaccine initiative.

Coronavirus disease 2019 (COVID-19)–related hospitalizations and deaths of children and teens are on the rise; vaccine experts warn against rushing a COVID-19 vaccine; 2 studies spotlighted the airborne spread of COVID-19.

The Unites States has its first reported case of coronavirus disease 2019 (COVID-19) reinfection; the FDA expands emergency use of remdesivir; experts lower the age for initial screening of colorectal cancer.

There is a certain element of antiscience sentiment in the United States and Europe that Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, finds troubling.

Every week, The American Journal of Managed Care® recaps the top managed care news of the week, and you can now listen to it on our podcast, Managed Care Cast.

President Donald Trump promised a coronavirus disease 2019 (COVID-19) vaccine by the end of the year; COVID-19 survivors report anxiety over reinfection; colleges release COVID-19 testing and quarantine data.

Coverage of our peer-reviewed research and news reporting in the healthcare and mainstream press.

NCCN added tafasitamab plus lenalidomide to their oncology practice guidelines for patients with relapsed or refractory DLBCL not otherwise specified.

Liquid CDX, a blood test, analyzes more than 300 genes and genomic signatures across all solid tumors.

Preliminary data find Moderna's COVID-19 vaccine effective in older adults; FDA authorizes use of $5 COVID-19 rapid test; the DOJ asks for nursing home data from 4 blue states.

CMS requires all nursing homes to test staff for coronavirus disease 2019; FEMA signals it may end reimbursement for personal protective equipment; CDC issues new exposure testing recommendations.

Scientists say data on the coronavirus disease 2019 (COVID-19) treatment convalescent plasma may be misrepresented; Anthony Fauci, MD, warned that distributing a COVID-19 vaccine without testing in large trials could affect other vaccines; remdesivir was shown to have little effect in patients hospitalized with COVID-19.

The FDA issued an emergency use authorization (EUA) for convalescent plasma to treat coronavirus disease 2019 (COVID-19); limited transmission of COVID-19 was reported in reopened childcare centers; universal mask mandates could save lives.

A proprietary algorithm can help payers identify patients at high risk of progressive chronic kidney disease.

Every week, The American Journal of Managed Care® recaps the top managed care news of the week, and you can now listen to it on our podcast, Managed Care Cast.

Some types of pain affect cognitive ability, including number sense, and yet some tools to assess pain use numerical scales. A recent study sought to determine if number sense is altered in patients with chronic and acute pain compared with healthy subjects.

Coverage of our peer-reviewed research and news reporting in the healthcare and mainstream press.
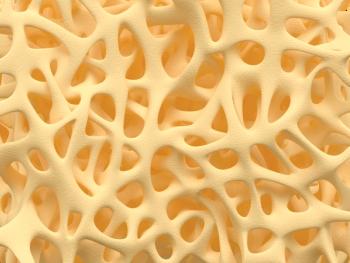
Patients with psoriasis and psoriatic arthritis are at a greater risk for osteopenia and osteoporosis, but the associate is the result of secondary factors, such as medication.

A new phase 1 trial will test aerosolized chemotherapy in patients with cancer; Uber and NimbleRx partner to deliver prescriptions; Missouri prisons see a rise in coronavirus disease 2019 (COVID-19) cases.

For multiple sclerosis, the therapy will be sold as Kesimpta by Novartis; it is administered by patients once a month through the Sensoready autoinjector pen.

Reports of child abuse fell due to the coronavirus disease 2019 (COVID-19) pandemic; higher levels of the new coronavirus were found in children than in hospitalized adults; Michigan will pay $600 million to Flint residents.

The FDA rejects Gilead’s new drug application for filgotinib; new survey results on mental health care access for LGBTQ youth; Anthony S. Fauci, MD, thinks a mandatory coronavirus disease 2019 (COVID-19) vaccination is unlikely.

The coronavirus disease 2019 (COVID-19) is now the third-leading cause of death in the United States; the FDA issued an alert for a COVID-19 test; promoting pediatric eye care as schools go virtual.

Satralizumab is the third drug to be approved by the FDA for neuromyelitis optica spectrum disorder (NMOSD).

Officials from health care and manufacturing predict ongoing shortages of personal protective equipment (PPE); the FDA authorized use of a saliva test for coronavirus disease 2019 (COVID-19); 1 in 3 Americans will not take a COVID-19 vaccine.

Evidence-Based Oncology regulatory updates for August 2020 feature coverage of Kite Pharma's new CAR T-cell therapy and selinexor in DLBCL.

Evidence-Based Oncology managed care updates for August 2020 feature coverage of liquid biopsies, companion diagnostics, and CAR T-cell therapy.

Every week, The American Journal of Managed Care® recaps the top managed care news of the week, and you can now listen to it on our podcast, Managed Care Cast.

Clinical updates for August 2020 featured in Evidence-Based Oncology.

Coverage of our peer-reviewed research and news reporting in the health care and mainstream press.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
