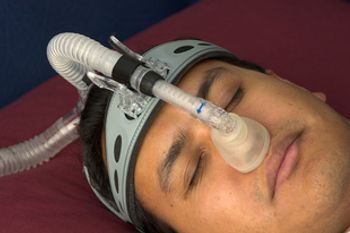
New York City health officials have declared an end to the city’s biggest measles outbreak in nearly 3 decades; Senator Bernie Sanders, I-Vermont, has proposed canceling $81 billion in existing medical debt; Vertex Pharmaceuticals is taking a chance on a start-up’s early-stage science that could potentially one day emerge as a functional cure for type 1 diabetes (T1D).