
Cancer care evolves with innovative therapies and financial challenges, emphasizing the need for equitable access and sustainable models in treatment delivery.

Cancer care evolves with innovative therapies and financial challenges, emphasizing the need for equitable access and sustainable models in treatment delivery.

A session explores how value-based oncology integrates pharmacy, precision medicine, and real-world evidence to enhance lung cancer care and patient outcomes.

UC Davis Health enhances patient care through a shared services center, ensuring continuity of care and optimizing pharmacy growth strategies.

Programs such as Meds to Bed can work in tandem with retail pharmacies to offer accessible medications for patients.

Timothy W. Cutler, PharmD, associate chief of ambulatory care at UC Davis Health, describes how pharmacy services patients healthy and out of the hospital.

Prior authorizations can affect both health system operations and worsen physician burnout.

The availability of retail pharmacies throughout the country have changed the health system in terms of accessibility and treatment, explains Mark Riggle, PharmD.
Updated results from the MARIPOSA trial show that amivantamab plus lazertinib significantly reduces EGFR- and MET-driven resistance compared with osimertinib.
Monthly SC amivantamab plus daily lazertinib demonstrated strong efficacy and tolerability in EGFR-mutated advanced NSCLC, providing a convenient alternative to IV dosing.
Central nervous system (CNS) metastases in EGFR-mutant non–small cell lung cancer (NSCLC) remain a major challenge, with emerging therapies and evolving trial designs aiming to improve outcomes and address unmet needs.
Uncommon EGFR mutations in non–small cell lung cancer (NSCLC) remain challenging to treat, but new tyrosine kinase inhibitors, bispecific antibodies, and a proposed “PACCage insert” framework provide opportunities to advance precision therapy.
Treatment of EGFR-mutated non–small cell lung cancer (NSCLC) is shifting toward next-generation sequencing and combination regimens that improve survival but increase toxicity, requiring individualized care.
Antibody-drug conjugates are rapidly reshaping the treatment landscape of non–small cell lung cancer (NSCLC), with advances in design, clinical efficacy, and regulatory approvals tempered by ongoing challenges in toxicity, resistance, and biomarker optimization.

Bispecific antibodies are emerging as a transformative class in advanced non–small cell lung cancer (NSCLC), with agents such as amivantamab and zenocutuzumab already demonstrating clinical benefit and a broad pipeline of investigational therapies showing promise in overcoming resistance.
National Comprehensive Cancer Network (NCCN), the European Society for Medical Oncology (ESMO), and the American College of Chest Physicians (CHEST) offer complementary yet distinct frameworks for lung cancer care, reflecting differences in evidence evaluation, regional adaptation, and policy integration.

Oranus Mohammadi, MD, outlines how antibody-drug conjugates are transforming breast cancer treatment across subtypes and discusses her approach to sequencing high-cost targeted therapies within payer and clinical practice constraints.

Oranus Mohammadi, MD, discusses the emerging applications of circulating tumor DNA (ctDNA) in breast cancer care and emphasizes the importance of clear communication to help patients navigate uncertain or anxiety-provoking biomarker test results.

Joanne Mortimer, MD, FACP, FASCO, discusses the practical applications and limitations of circulating tumor DNA (ctDNA) testing in breast cancer, highlighting its role in guiding targeted therapy, challenges in patient communication and payer coverage, and unique barriers for male patients.

Jorge Nieva, MD, explores the challenges of translating biomarker testing into treatment decisions for non–small cell lung cancer (NSCLC), the role of repeat testing in detecting resistance mutations, and the importance of equitable access to molecular diagnostics in value-based care settings.

Jorge Nieva, MD, highlights the critical role of molecular testing in non–small cell lung cancer (NSCLC) care, while addressing barriers such as limited tissue samples, delayed turnaround times, and the need for faster, more accessible diagnostic technologies.

Yale Podnos, MD, MPH, FACS, discusses strategies to address social determinants of health in oncology, improve clinical trial enrollment for underserved communities, and leverage value-based care models to reduce financial toxicity and ensure equitable cancer care.

Daniel Virnich, MD, highlights the need for proactive social determinants of health screening, language-inclusive clinical trial practices, value-based treatment decisions, and policy reforms to improve equitable access to cancer care.

Lauren Antrim, MD, of City of Hope Cancer Center Duarte, emphasized the need for more evidence to guide optimal immunotherapy duration and sequencing in in non–small cell lung cancer (NSCLC), highlighting ongoing trials and the potential role of ctDNA in tailoring treatment strategies.

Lauren Antrim, MD, emphasized the need to balance safety, efficacy, and financial considerations when managing immune checkpoint inhibitors in NSCLC, underscoring the importance of patient-centered discussions and ongoing trials to refine treatment duration strategies.

Jonathan Thompson, MD, MS, explains how financial, insurance, and socioeconomic barriers limit equitable access to biomarker testing and advanced therapies, underscoring the need for provider advocacy and systemic support.

In advanced non–small cell lung cancer (NSCLC), discontinuing immunotherapy after 2 years can maintain durable responses while reducing financial and toxicity burdens, with decisions guided by residual disease testing and shared decision-making, explained Jonathan Thompson, MD, MS.
Jonathan Thompson, MD, MS, explains that adjuvant immunotherapy benefits patients with early-stage lung cancer with incomplete neoadjuvant response, while treatment decisions in the adjuvant setting must weigh efficacy, toxicity, and limited evidence.
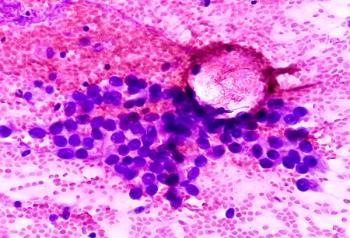
Jonathan Thompson, MD, MS, highlighted that reducing delays in molecular testing and treatment initiation is critical for improving lung cancer outcomes, and that clinical trial data suggest immunotherapy duration can often be safely de-escalated in patients who achieve a complete pathologic response.

Jenny Craven, PharmD, BCPS, outlines the strategies UC Davis Health has used to successfully navigate the complex challenges of implementing cell and gene therapies.

Health systems must prepare for the growing impact of cell and gene therapies by addressing their high costs, complex care pathways, and payer collaboration needs to ensure timely and sustainable patient access.
Published: September 6th 2025 | Updated:

Published: August 29th 2025 | Updated:

Published: March 18th 2023 | Updated:
Published: September 6th 2025 | Updated:

Published: August 15th 2025 | Updated:

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
