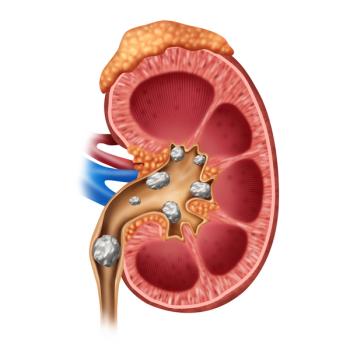
More than a year into the COVID-19 pandemic, a new study suggests that individuals recovering from acute kidney injury stemming from COVID-19 will require kidney monitoring after they leave the hospital.
Allison is Associate Editorial Director for The American Journal of Managed Care® (AJMC®) and The Center for Biosimilars®. She joined AJMC® in 2017. She produces and oversees written, video, and podcast content across several disease states and issues surrounding value-based care and health policy.
She has an MPA from New York University. You can connect with Allison on LinkedIn.

More than a year into the COVID-19 pandemic, a new study suggests that individuals recovering from acute kidney injury stemming from COVID-19 will require kidney monitoring after they leave the hospital.

In a briefing, the White House COVID-19 Team discussed the first guidance from the CDC on activities that individuals fully vaccinated against COVID-19 can safely resume, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, gave an update on antiretroviral drug development.
A retrospective study looking at pediatric patients hospitalized in 4 New York hospitals in 2020 with COVID-19 or multisystem inflammatory syndrome in children (MIS-C) found that acute kidney injury (AKI) occurred in 11.8% of patients.

Despite benefitting from organ transplants similarly to other patients with kidney failure, a new study found patients with kidney failure associated with sickle cell disease (SCD) are less likely to receive transplants.
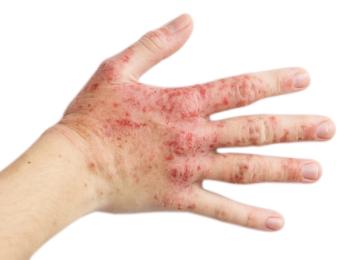
Despite the level of disease severity of atopic dermatitis, children with the condition usually have at least 1 other inflammatory or atopic comorbidity, according to new study results.

Most orphan drug spending does not actually go to patients with rare diseases, a new study shows.
The FDA granted emergency use authorization (EUA) for the distribution of the nation's third COVID-19 vaccine, and on Sunday, a CDC panel will meet to decide on recommendations to put it into practice.
More than 2 years after treatment, some of the patients with relapsed/refractory multiple myeloma have yet to see a relapse. An FDA decision on the therapy is expected within a month.

Use of a specialty pharmacy to take over the management of prior authorization (PA) requests for a dermatology practice significantly reduced the time to a decision and also decreased the time it took to fill the medication.

An FDA advisory panel Friday unanimously endorsed the third US COVID-19 vaccine, a 1-shot vaccine produced by Janssen, a subsidiary of Johnson & Johnson (J&J), and an emergency use authorization by the FDA is expected to follow shortly.

The results also showed that the infants achieved certain milestones in motor development, pulmonary function, and survival.

Multimorbidity affects 1 in 4 US adults and leads to increased health care use and lower quality of life.
The results from an analysis of patients from 12 rheumatology centers treated with secukinumab for psoriatic arthritis were in line with earlier pivotal trials, the researchers said.

Planning and education can help create systemwide change in order to implement greater use of advance care planning and palliative care for those with end-stage renal disease (ESRD).

The Department of Justice (DOJ), under a new administration, on Wednesday dropped its previous position that the now-defunct tax provision in the Affordable Care Act (ACA) cannot be severed from the rest of the law, thus making the entire health law unconstitutional.

Compared with pollen season in 1990, today's pollen season is starting earlier and lasting longer, largely because of human-caused climate change, researchers said.

A recent study took a look at the use of biologics for generalized pustular psoriasis, a rare, potentially life-threatening inflammatory disorder.

The findings suggest that this new categorization of cognitive deficits in multiple sclerosis may support clinicians in treatment choices and help tailor cognitive rehabilitation strategies, the authors said.

The authors said this study included more up-to-date definitions of diagnosing pulmonary arterial hypertension (PAH), which is an increasing factor in deaths from systemic sclerosis (SSc).

The Biden administration will reopen the health exchanges created by the Affordable Care Act (ACA); direct HHS and other agencies to reexamine other health policies, including Medicaid work requirements; and reverse the so-called global gag rule while affirming support for reproductive health.
The study sought to understand how psoriasis treatments perform in real-world settings.

Patients with rheumatoid arthritis (RA) were asked their preferences of second-line treatment options such as biologics and Janus kinase inhibitors, taking into account such factors as the probability of severe side effects, treatment effectiveness, administration route, and more.

As the nation crossed the threshold of 400,000 deaths from the pandemic, the Biden transition team said that one of his first actions will be an executive order calling on Americans to do “their patriotic duty” and wear a mask.
The statement is aimed at drug companies, policymakers, pharmacy benefit managers, employers, and others, and calls to attention soaring insulin costs, which were the subject of several Congressional hearings in 2019.

Two story lines predominated renal news in 2020: coronavirus disease 2019's effect on the kidneys and the overwhelming benefits of sodium-glucose cotransporter 2 inhibitors for those with chronic kidney disease and type 2 diabetes.

Researchers sought to gain a better understanding of the relationship between genetic mutations in metastatic colon cancer (mCRC), advanced disease, and locations of primary tumors.

The finding about mindfulness-based stress reduction (MBSR) was a secondary outcome of the double-blinded, randomized, placebo-controlled clinical trial. The primary outcome—a reduction in the frequency of migraines—was not reached.
As a neurodegenerative disease, secondary progressive multiple sclerosis (SPMS) may lead to a degradation of cognition skills as the disease progresses.

The FDA Friday issued the second Emergency Use Authorization (EUA) for a coronavirus disease 2019 (COVID-19) vaccine, this one to Moderna, as the pandemic reached critical stress points across the country, especially in California.

After a daylong meeting, an FDA advisory panel agreed that the second vaccine in the United States to prevent coronavirus disease 2019 (COVID-19) should be put forward for use.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
