
The FDA has granted approval to pembrolizumab for the treatment of adult patients with resectable, locally advanced HNSCC whose tumors express PD-L1 with a CPS of ≥1.
Jordyn Sava is an assistant editor for Targeted Oncology™.

The FDA has granted approval to pembrolizumab for the treatment of adult patients with resectable, locally advanced HNSCC whose tumors express PD-L1 with a CPS of ≥1.
Dosing has started in a clinical evaluating peluntamig, which targets DLL3 and CD47, combined with chemotherapy in patients with DLL3-expressing small cell lung cancer and neuroendocrine carcinoma.

The CELESTIAL-301 trial evaluating SynKIR-310, a novel chimeric antigen receptor T-cell therapy, has dosed its first patient with relapsed/refractory B-cell non-Hodgkin lymphoma (B-NHL).
The FDA approved a phase 3 trial to assess amezalpat with the current standard of care in unresectable or metastatic hepatocellular carcinoma.

The key secondary end point of overall survival (OS) was met in the DREAMM-7 trial of belantamab mafodotin (Blenrep; GSK) for the treatment of patients with relapsed/refractory multiple myeloma (R/R MM).

NXP800 was granted orphan drug designation from the FDA in ARID1a-deficient ovarian, fallopian tube, and primary peritoneal cancers.
The FDA granted orphan drug designation for elraglusib, a novel drug for treating advanced soft tissue sarcoma.

The phase 3 inMIND trial evaluating tafasitamab in combination with lenalidomide and rituximab in relapsed or refractory follicular lymphoma showed promising progression-free survival findings, according to topline results.
Revumenib, a first-in-class Menin inhibitor, was granted priority review from the FDA for the treatment of adult and pediatric relapsed or refractory KMT2A-rearranged acute leukemia.

David J. Andorsky, MD, board-certified medical oncologist and hematologist at Rocky Mountain Cancer Centers, discussed findings from a study on patterns of Bruton tyrosine kinase (BTK) inhibitor care use and social determinants of health (SDOH) among patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).

Andrew Srisuwananukorn, MD, of the Ohio State University Comprehensive Cancer Center, explained the potential of artificial intelligence (AI)-based support tools for differentiating primary myelofibrosis (prePMF) and essential thrombocythemia (ET) in the community setting.

Positive data from the phase 3 EV-302 trial of enfortumab vedotin with pembrolizumab in locally advanced or metastatic urothelial cancer have shifted the landscape.

Rhenium (186Re) obisbemeda (Plus Therapeutics) shows potential to address the unmet need of new treatment options for patients with breast cancer and leptomeningeal metastases and will continue to be evaluated in the ReSPECT-LM program. The orphan drug designation (ODD) was granted on November 3.
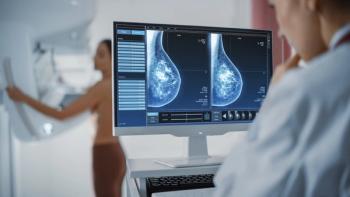
The dual primary end points of overall survival and progression-free survival were encouraging when patients with hormone receptor–positive (HR+), HER2-negative (HER2–) breast cancer were treated with datopotamab deruxtecan.
A recent study suggests that patients with myelodysplastic syndrome (MDS) could potentially benefit from allogeneic hematopoietic cell transplantation (HCT) regardless of genetic mutation status.
Patients with RET fusion-positive advanced or metastatic non–small cell lung cancer had a survival benefit when treated with selpercatinib in the LIBRETTO-431 study.

Data from 2 studies, LIGHTHOUSE and SPOTLIGHT, have shown the benefit of flotufolastat F 18 injection as a radiohybrid PSMA-targeted PET imaging agent in prostate cancer. The agent has now been added to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology.

In an interview with Targeted Oncology, Sagun Shrestha, MD, evaluated new developments and clinical trials for patients with extensive-stage small cell lung cancer.

Coverage of research presented by Strategic Alliance Partners: OneOncology, Florida Cancer Specialists & Research Institute, and The US Oncology Network.

Coverage of sessions on disparities in access to clinical trials and cancer screening, as well as relationships each to outcomes. A joint session presented by ASCO and the European Society for Medical Oncology (ESMO) highlighted progress and lingering gaps in lung and colorectal screening.

Results included those for several bispecifics, used as monotherapy and in combination, including early results for 2 bispecifics used together.

Results include a landmark study that involved seamless collaboration across adult and pediatric patient groups, leading to a highly diverse study population. Other coverage addresses access to novel therapies and what's coming in the pipeline, including CAR T-cell therapy with tyrosine kinase inhibitors.

Coverage of clinical trial results presented across a range of solid tumor cancers, including breast, ovarian, colorectal, and non-small cell lung cancer, among other types; as well as important findings in genomic testing.

Following positive phase 2 results from the ROSEWOOD study, BeiGene is seeking FDA approval of zanubrutinib plus obinutuzumab for select patients with relapsed/refractory (R/R) follicular lymphoma.

Barb Kunz, MS, LCGC, senior genetic counselor at the US Oncology Network, discussed the importance of germline genetic testing in patients with triple-negative breast cancer and other cancer types.

Jason Westin, MD, MS, FACP, director of the Lymphoma Clinical Research Program at the University of Texas MD Anderson Cancer Center, gave insight the ZUMA-7 trial of axicabtagene ciloleucel (axi-cel) in relapsed or refractory large B-cell lymphoma (R/R LBCL) and the study's implications in the broader LBCL landscape.

Judy Wang, MD, medical oncologist and clinical trials investigator at Sarah Cannon Research Institute at Florida Cancer Specialists & Research Institute, discussed the mechanism of action and rationale for studying CLN-619, an anti-MICA/B antibody, with and without pembrolizumab in patients with solid tumors.

Bhagirathbhai R. Dholaria, MD, assistant professor of medicine in the Department of Hematology-Oncology at Vanderbilt University Medical Center, discussed findings from the phase 2 TRiMM-2 trial of talquetamab plus daratumumab in multiple myeloma.

Barb Kunz, MS, LCGC, senior genetic counselor at the US Oncology Network, shared insight on a study of social determinants of health in the context of germline genetic testing for triple-negative breast cancer (TNBC) in the community oncology setting.

Dennis Slamon, MD, PhD, lead investigator on the NATALEE trial of ribociclib plus hormonal therapy in hormone receptor–positive, HER2-negative early-stage breast cancer, discussed the study's broad population and potential payer reactions.

Published: July 31st 2023 | Updated:

Published: June 5th 2023 | Updated:

Published: July 31st 2023 | Updated:

Published: July 31st 2023 | Updated:

Published: December 11th 2023 | Updated:

Published: July 31st 2023 | Updated:

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
