
Coverage of our peer-reviewed research and news reporting in the health care and mainstream press.

Julia is an associate editor for The American Journal of Managed Care® (AJMC®) and joined AJMC® in 2022. She produces written and video content covering multiple disease states, and assists in the screening process for manuscripts submitted to AJMC®.
She has a BA in English language and literature from Rutgers University. You can connect with Julia on LinkedIn.

Coverage of our peer-reviewed research and news reporting in the health care and mainstream press.

Lurbinectedin has been approved to be used in combination with atezolizumab or atezolizumab and hyaluronidase.

Louisiana and Mississippi had the highest rates of maternal and child mortality in the country, respectively.

Adverse events related to the immune system tended to accumulate over time when patients used immune checkpoint inhibitors (ICIs) over time.

Programs such as Meds to Bed can work in tandem with retail pharmacies to offer accessible medications for patients.

Several actions taken by the current administration may affect the landscape of prevention of HIV, prompting an analysis of these effects.

Despite an executive order aimed at addressing homelessness in the US, defunding vital programs could spell difficulty in helping the homeless.

Prior authorizations can affect both health system operations and worsen physician burnout.

Ana Baramidze, MD, head of the Department of Clinical Researches at the Todua Clinic in Tbilisi, Georgia, explained how clinicians can decide how to cemiplimab, which has a low discontinuation rate.

The executive order signed in July features policy shifts that would put states in charge of funding for encampment sweeps, among other policy shifts.
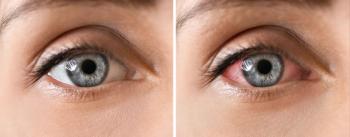
Children who had chronic, early-onset, nonanterior uveitis were at an increased risk of developing other complications surrounding the eye.

Any potential reduction in access to pre-exposure prophylaxis (PrEP) would increase both cost and the number of diagnoses of HIV.

The availability of retail pharmacies throughout the country have changed the health system in terms of accessibility and treatment, explains Mark Riggle, PharmD.

Funding cuts to childhood cancer research have put the subspecialty in a precarious position during Childhood Cancer Awareness Month.

The ease of acquiring prior authorizations for a new formulary drug plays a part in evaluating oncology drugs that are new to the market.

Cemiplimab demonstrated a 56% overall survival rate, making it a potential option for physicians to use for treating non–small cell lung cancer (NSCLC).

The approval allows all patients 12 years and older to use pembrolizumab and berahyaluronidase alfa-pmph for subcutaneous injection for solid tumor indications approved for intravenous pembrolizumab.

Despite higher burden of metabolic issues, individuals with HIV were not more metabolically ill.

Accessibility to gender-affirming surgery needs to be improved for transgender, nonbinary, and gender-diverse individuals.
The governors California, Oregon, Washington, and Hawaii came together to announce their winter virus recommendations.
Net health care costs would also increase by billions of dollars should pre-exposure prophylaxis become less accessible.

New demands to follow the most favored nation executive order could lead to some compliance but it is unknown just how effective they will be across all health care.

Although children living with myopia taking atropine did experience an increased incidence of cataracts, glaucoma, or maculopathy, it is unclear if this risk was confounded by myopia severity.

A reduction in HIV incidence is possible with more structured choices for pre-exposure and postexposure prophylaxis being offered to patients at risk of HIV.

Cemiplimab plus chemotherapy was found to be consistently effective in treating non-small cell lung cancer (NSCLC) and held up in the EMPOWER-Lung 3 trial.

Experts in cardiometabolic conditions gathered in Aurora, Colorado, on August 19, 2025, to discuss the methods available to prevent cardiovascular events.

Coverage of our peer-reviewed research and news reporting in the health care and mainstream press.

Making sure that children have access to high-quality care and parents trust that care is important in making sure that children are cared for after Medicaid cuts take effect.
Bone density was found to be lower in those diagnosed with HIV vs those who were HIV-negative.

DeepSeek-R1 was able to improve diagnosis management in these subspecialties while also lowering operation costs.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
