
A real-world study confirmed that the rate of adverse events associated with first-line nivolumab plus iplimumab therapy was in line with the safety profiles of other immuno-oncology and chemotherapy combination regimens.

Skylar is an associate editor for The American Journal of Managed Care® (AJMC®) and The Center for Biosimilars®, and joined AJMC® in 2020. She is responsible for covering all aspects of the ever-changing global biosimilar industry and produces content that is accessible and informative for all health care stakeholders.
She has a BA in journalism and media studies from Rutgers University. You can connect with Skylar on LinkedIn.

A real-world study confirmed that the rate of adverse events associated with first-line nivolumab plus iplimumab therapy was in line with the safety profiles of other immuno-oncology and chemotherapy combination regimens.

Mike Brown, vice president of managed services at Cardinal Health, discusses Cardinal Health's goals to to leverage technology, while reducing costs to the health care system.

Mike Brown, vice president of managed services at Cardinal Health, discusses how remote pharmacy services are revolutionizing patient care by improving accessibility and medication safety.

Abstracts from the 65th American Society of Hematology (ASH) Annual Meeting and Exposition provided new insight into multiple treatments for patients with multiple myeloma (MM), chronic lymphocytic leukemia (CLL), and follicular lymphoma (FL).
Researchers found that venetoclax, either alone or post treatment with a Bruton tyrosine kinase inhibitor (BTKi), was safe and effective for patients with chronic lymphocytic leukemia (CLL).

Mike Brown, vice president of managed services at Cardinal Health, shares how drug cost control software has improved monitoring efficiency and reduced drug spending.

Mike Brown, vice president of managed services at Cardinal Health, discusses how Cardinal Health has worked to integrate key digital health solutions among hospitals across the United States.
Health care workers who received SARS-CoV-2 vaccinations were found to be protected against declines in respiratory parameters after overcoming COVID-19, highlighting the importance of COVID-19 vaccines for health care workers.

A recent study encapsulates how patients and caregivers characterize the impact of Duchenne muscular dystrophy (DMD) on patients’ physical limitations and symptom burden, potentially helping to inform patient-centered strategies for assessing clinical outcomes in DMD research.

Patients with spinal muscular atrophy (SMA) treated with a more expensive medication were found to have higher pharmacy costs but lower SMA-related health care resource utilization and medical costs compared with patients receiving standard-of-care nusinersen monotherapy.
A recent study found that uptake of disease-modifying therapies (DMTs) has been low among patients with sickle cell disease, suggesting that more interventions that consider individual patient characteristics are needed to improve adoption.

A study investigating potential subtypes of idiopathic hypersomnia (IH) found that patients with IH who experience unrefreshing naps have less fragmented sleep compared with those who take refreshing naps, suggesting unrefreshing naps could serve as a supportive IH clinical feature.

A new survey from the National Alliance of Healthcare Purchaser Coalitions found that employers are planning to place a larger focus on obesity management, women’s health, and health equity in 2024 and beyond.

Bio-Thera Solutions’ Avzivi (bevacizumab-tnjn) has become the fifth biosimilar referencing Avastin (bevacizumab) to be approved in the United States.

A population-based study found that out-of-hospital cardiac arrest events resulting from drug overdoses have significantly increased from 2015 to 2021, particularly among patients who have taken a combination of stimulants and opioids.

Depression symptoms are often underdiagnosed and undertreated in patients with psoriasis and psoriatic arthritis, contributing to a worsened quality of life (QOL). However, a recent study found that depression, functional impairment, and worsened QOL are related.

The presence of cochlear dysfunction found in patients with alopecia areata (AA) suggests the need for more comprehensive assessment and management of hearing-related issues associated with AA.

A cohort study found that early changes in patient-reported outcomes (PROs) were associated with treatment response and survival outcomes in patients with advanced gastrointestinal cancer, supporting the need for more routine implementation of PRO monitoring in cancer care spaces.

A new report from IQVIA provides an overview of current US drug shortages, shedding light on major areas of concern, such as medications to address pain, cardiovascular conditions, obesity and diabetes, and multiple forms of cancer.

Dr. Parth Rali, MD, of Temple University Hospital explained the challenges of managing patients with intermediate-risk pulmonary embolism (PE) and how risk stratification tools can help to address these challenges.

Health equity coverage appearing in the October 2023 issue of Evidence-Based Oncology.

Phase 3 data presented at ISPAD found that teplizumab-mzwv (Tzield) was better at slowing the progression of type 1 diabetes (T1D) in newly diagnosed children and adolescents.
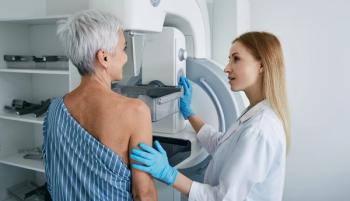
No differences in mammography completion were observed between an automated opt-out vs an opt-in patient outreach program for breast cancer screening, according to a randomized controlled trial. However, differences in administration burden between programs could lead clinics to prefer one over the other.
A study assessing an experimental cell-free DNA myelination liquid biopsy test was found to be 91% accurate at identifying early-stage epithelial ovarian cancer.

Researchers found that heated tobacco products can reduce risks of exacerbations and exposure to toxic chemicals compared with combustible cigarettes in patients with chronic obstructive pulmonary disease (COPD).

Operational forecasting for patients who are eligible for gene therapies will be crucial for addressing the accessibility and affordability challenges with these agents, especially as more gene therapies are expected to enter the market in the next few years.

During a session at AMCP Nexus, panelists provided a historical overview on the 340B drug discount program, highlighting the intentions of the plan and the research gaps leading to misconceptions about the program and whether it’s working to lower costs for patients.

Speakers at AMCP Nexus 2023 reviewed the major changes resulting from the Inflation Reduction Act (IRA) that impacted Medicare in 2023 and previewed the new provisions being implemented in the coming years.

At AMCP Nexus, Evernorth's Aimee Tharaldson, PharmD, provided an overview of which medications the FDA has approved in 2023 and what’s to come in nononcology areas over the next 5 years, including new drugs for Alzheimer disease, hemophilia, and nonalcoholic steatohepatitis (NASH).

Panelists at AMCP Nexus provided an overview of the US obesity epidemic, dispelling myths about the causes of obesity, highlighting racial and economic disparities across the nation, and tackling how the managed care space can ensure accessibility to care for all patients.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
