
The American Journal of Managed Care®’s Strategic Alliance Partnerships provide valuable insights into health care trends through collaborations with health systems, payers, oncology practices, and more.

Skylar is an associate editor for The American Journal of Managed Care® (AJMC®) and The Center for Biosimilars®, and joined AJMC® in 2020. She is responsible for covering all aspects of the ever-changing global biosimilar industry and produces content that is accessible and informative for all health care stakeholders.
She has a BA in journalism and media studies from Rutgers University. You can connect with Skylar on LinkedIn.

The American Journal of Managed Care®’s Strategic Alliance Partnerships provide valuable insights into health care trends through collaborations with health systems, payers, oncology practices, and more.

The top 5 most-viewed videos of 2024 highlighted advancements in therapies, heart disease screening, pediatric palliative care, medication disruptions, and the role of economic research in health policy.

Ryan Nguyen, DO, physician and researcher at the University of Illinois Chicago, emphasizes the transformative impact of immunotherapy in non–small cell lung cancer (NSCLC) and the need for precise biomarkers and equitable access to advanced treatments through systemic testing protocols.

Coverage from the Institute for Value-Based Medicine event in Chicago, Illinois.

Recent discussions at an Institute for Value-Based Medicine event highlighted the significant potential of biosimilars in reshaping the health care landscape, despite facing considerable barriers to adoption.

Divya Gupta, MD, assistant professor at the Northwestern University Feinberg School of Medicine, emphasized the transformative role of biomarker-driven therapies in advancing non–small cell lung cancer (NSCLC) treatment and highlighted the critical importance of multidisciplinary collaboration.

The approval of Yesintek (ustekinumab-kfce), the sixth biosimilar to reference Stelara, will be used to treat patients with various immunology conditions, including psoriatic arthritis and inflammatory bowel disease.

Matias Sanchez, MD, assistant professor in the Department of Medicine at the University of Illinois Chicago, discussed recent advancements in multiple myeloma treatment, including the potential of cell therapies and bispecific antibodies.

Sandra Cuellar, PharmD, from the University of Illinois Chicago, highlights the critical role of real-world data in shaping reimbursement models for oncology therapies and emphasizes the growing importance of patient-centered care through shared decision-making, precision medicine, and patient-reported outcomes.

Matias Sanchez, MD, assistant professor in the Department of Medicine, Division of Hematology and Oncology, University of Illinois Chicago, emphasizes the importance of patient education and caregiver involvement in managing complex therapies and advises oncologists to confidently integrate advanced treatments.

Divya Gupta, MD, assistant professor at Feinberg School of Medicine at Northwestern University, discussed the potential of using circulating tumor DNA and minimal residual disease (MRD) assays for personalized treatment in non–small cell lung cancer (NSCLC), while emphasizing the unmet need for effective second-line therapies for patients without driver mutations.

President-elect Donald Trump will nominate heart surgeon turned television personality and politician Mehmet Oz, MD, also known as Dr Oz, to lead CMS.

Oncology pharmacists play a vital role in managing complex outpatient therapies by assessing patient suitability, educating patients, and implementing protocols to ensure safe and effective treatment.

A 25-year study of the Lothian Birth Cohorts found that childhood intelligence, lifestyle factors, and brain white matter health significantly impact cognitive aging and longevity, highlighting the importance of consent, ethics, and biological sample collection in ongoing research.

A study finds that telehealth does not lead to an increase in wasteful, low-value care and may even reduce unnecessary tests and procedures.

In a Q&A, an FDA spokesperson discusses efforts to reduce misinformation about biosimilars through education, the agency’s collaboration with global regulators to streamline development, and its work to address drug shortages while emphasizing safety, efficacy, and public trust.

A panel of industry experts discussed the complexities of biosimilars and interchangeability, emphasizing the challenges in adoption, the need for regulatory and legislative solutions, and the importance of education to combat misinformation.
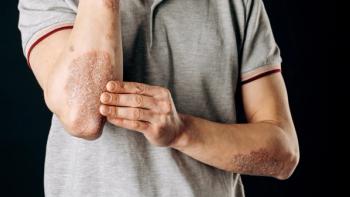
Individuals with self-reported moderate to severe psoriatic arthritis (PsA) face a higher disease burden, particularly among Black participants, highlighting the need for a better understanding of the interplay between PsA severity and racial and ethnic disparities.


Imuldosa (ustekinumab-srlf) is the fifth ustekinumab biosimilar referencing Stelara to receive regulatory approval in the US.

Value-based agreements come with risks and benefits, but a health system’s existing facilities and initiatives can help support the goals associated with them, JT Lew, PharmD, MBA, a managed care pharmacist at MultiCare Health System, explained.

Ted Okon, MBA, executive director of the Community Oncology Alliance, spoke with The American Journal of Managed Care® about how the Federal Trade Commission's (FTC) lawsuit against pharmacy benefit managers (PBMs) could affect the future of pharmaceuticals and oncology.

JT Lew, PharmD, MBA, managed care pharmacist at MultiCare Health System, spoke to the impacts of processes such as prior authorization and step therapy requirements in the realm of multiple sclerosis (MS).

The Federal Trade Commission (FTC) has filed a legal complaint accusing the 3 largest pharmacy benefit managers (PBMs) in the US—Caremark, Express Scripts, and Optum Rx—of inflating insulin costs for patients, prompting renewed calls for PBM reform.
The FDA approval of amivantamab-vmjw (Rybrevant) in combination with chemotherapy is the first targeted treatment to cut disease progression risk for EGFR-positive non–small cell lung cancer (NSCLC).

Express Scripts is suing the Federal Trade Commission (FTC), demanding the retraction of a report it claims is false and harmful to the pharmacy benefit manager (PBM) industry.

The first overall survival analysis of the KEYNOTE-522 trial showed positive outcomes with neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab monotherapy vs neoadjuvant chemotherapy alone in high-risk early-stage triple-negative breast cancer (TNBC).

Policy changes, such as banning spread pricing and promoting transparency, are necessary to realign the pharmacy benefit manager (PBM) market and ensure that health care resources benefit patients and providers rather than being diverted by middlemen, according to panelists at the Community Oncology Alliance Payer Exchange Summit.

The need for improved collaboration between payers and providers is key to successfully implementing value-based care initiatives that address patient needs, ensure measurable outcomes, and overcome challenges, according to panelists at the Community Oncology Alliance Payer Exchange Summit.

A panel discussion at the Community Oncology Alliance (COA) Payer Exchange Summit highlighted the tension between state regulation of pharmacy benefit managers (PBMs) and the Employee Retirement Income Security Act (ERISA) preemption, emphasizing the need for reforms to balance employer uniformity with addressing PBM practices.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
