Biosimilars
Latest News
Latest Videos

CME Content
More News

Neal Dave, PharmD, the executive director of pharmacy at Texas Oncology, discussed physician anticipation for the first biosimilars for programmed death (PD)-1 inhibitors.

Switching from Herceptin (reference trastuzumab) to trastuzumab biosimilars could significantly reduce direct medication costs attributed to breast cancer management in Saudi Arabia, researchers concluded.

Neal Dave, PharmD, the executive director of pharmacy at Texas Oncology, talks about the anticipation for more biosimilars to enter the oncology market and the current level of interest in the pipeline.

In comparison with reference rituximab (Rituxan), the use of rituximab biosimilars in combination therapy produced similar 3-year overall survival among patients with large-B-cell lymphoma (DLBCL), investigators concluded.

Although previous work has found lower costs for biosimilar filgrastim compared with reference filgrastim, this study found that site of care can change this calculus, reducing savings.

Neal Dave, PharmD, the executive director of pharmacy at Texas Oncology, discusses the potential for biosimilar utilization to grow and the reasons why his practice favors them over reference products.
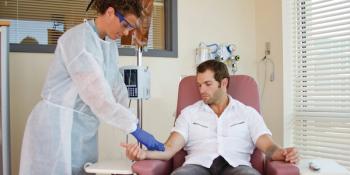
Switching patients from reference pegfilgrastim to a biosimilar could lead to substantial cost savings, with potential to offset the cost of and allow for greater access to chemotherapy treatment, investigators concluded.
A 2020 trends report from Artemetrx showed how biosimilar competition has contributed to lower average sales prices and claim percentages for originator products, suggesting that biosimilars are making a difference despite slow uptake.

Vizient recently released its Summer 2021 Pharmacy Market Outlook, which projects pharmaceutical spending for 2022 based on an analysis of its members' data. On this episode of Managed Care Cast, we talk about some of the findings.

Neal Dave, PharmD, the executive director of pharmacy at Texas Oncology, talks about the importance of conducting regular evaluations to ensure that prescribing the most cost-effective option, whether that be a biosimilar or the reference product.

In one of the first studies to examine real-world evidence on rituximab biosimilars in the United States, investigators found that many providers treating patients with non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL) are regularly prescribing a biosimilar rituximab.
Gary Lyman, MD, MPH, an oncologist and hematologist, discusses how financial incentives, concerns for patients, and interchangeability impact biosimilar prescribing patterns.

Two oncologists dive into the reasons behind physician hesitancy and unwillingness to prescribe biosimilars to patients with cancer and make suggestions on how to increase confidence in these agents.

Gary Lyman, MD, MPH, an oncologist and hematologist, compares the uptake of filgrastim biosimlars with pegfilgrastim biosimilars.

Amgen has received a letter from the FDA cautioning it against making misleading claims in advertising about its pegfilgrastim injector.

Gary Lyman, MD, MPH, an oncologist and hematologist, gives his thoughts on whether health care institutions need to be doing more to encourage use of biosimilars.
The price of reference products reduced each time a new biosimilar was added to a drug program’s reimbursement list, supporting the notion that reimbursing biosimilars creates more competition and lowers drug costs, according to a Polish study.

Gary Lyman, MD, MPH, an oncologist and hematologist, discusses how biosimilars have fared in the oncology space compared to other therapeutic areas.

Twenty-eight patient advocacy groups wrote a letter pledging endorsement of the Bolstering Innovative Options to Save Immediately on Medicines (BIOSIM) Act, a bill aimed at increasing reimbursement and incentivizing prescriptions for biosimilars.

Ivo Abraham, PhD, RN, a professor with the University of Arizona Health Sciences in the Department of Pharmacy Practice, discusses how the COVID-19 pandemic is likely to impact oncology biosimilar utilization.

A report from Xcenda showed that biosimilars for 8 blockbuster reference biologics have successfully kept drug prices from increasing by an average of 56%, restoring the possibility that biosimilars could achieve significant discounts despite facing several barriers to uptake.


A study examining how US biosimilar uptake has evolved found that biosimilars launched more recently have seen better uptake than biosimilars that have been on the market for a longer period.
Celltrion Healthcare’s high-concentration adalimumab biosimilar demonstrated comparable safety, efficacy, and immunogenicity profiles to the reference product for up to a year, providing further evidence supporting use of the product.
Sandoz Canada launches its sixth biosimilar, an anticoagulant used to treat deep vein thrombosis, on the Canadian market.