Non-Small Cell Lung Cancer
Latest News

Latest Videos

More News

Outcomes among patients with stage IV non–small cell lung cancer as evaluated within clinical trials via Response Evaluation Criteria in Solid Tumors (RECIST) and clinician response criteria in observational studies were compared for their concordance and reliability.
Consistent with previous observations, overall survival and progression-free survival remained strong at the follow-up point.
This analysis evaluated patient-related outcomes between those who did and did not receive consolidation durvalumab after chemoradiotherapy for unresectable stage III non–small cell lung cancer.

For patients with early-stage non–small cell lung cancer (NSCLC), combining neoadjuvant immune checkpoint inhibitors and platinum-based chemotherapy improves 2-year outcomes over chemotherapy alone, suggest findings of an extensive literature review and meta-analysis.

Seeking more data on adjuvant alectinib in patients who have resectable ALK-positive non–small cell lung cancer (NSCLC), investigators compared outcomes vs chemotherapy.

The data for this analysis on survivors of lung cancer came from Johns Hopkins Sidney Kimmel Comprehensive Cancer Center thoracic oncology clinics.

Authors conducted a systematic literature review to gather evidence on the appropriateness of recommended treatments for locally advanced non–small cell lung cancer (LA-NSCLC) from the American Radium Society Appropriate Use Criteria Thoracic Committee.
The study compared the adverse events and surgical, pathological, and efficacy outcomes associated with neoadjuvant chemoimmunotherapy vs chemotherapy, particularly focusing on patients with PD-L1 levels less than 1%.

This analysis found the complex decision-making process of lung cancer screening among eligible individuals is infused with 3 principal themes that may influence how primary care physicians approach patients with comorbidities.

This analysis included 119 patients with advanced lung cancer, who were evaluated on 3 facets of physical activity over 14 days of using the amuelink wearable device from Sony: metabolic equivalent tasks, distance walked, and steps taken.

Investigators examined the downstream effect of single-gene testing for guideline-recommended biomarkers on comprehensive genomic profiling in non–small cell lung cancer (NSCLC).
Overall rates for lung cancer screening remain low, despite the US Preventive Services Task Force revising its annual screening recommendation in 2021 by lowering the age of initial screening for current or former smokers and their pack-year history.
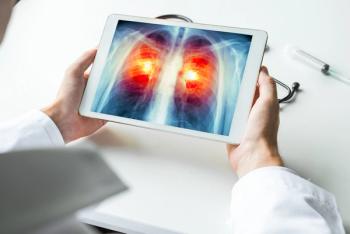
A review explores the evidence supporting the use of circulating tumor DNA (ctDNA) liquid biopsies to help direct the evaluation and management of EGFR-mutated non–small cell lung cancer (NSCLC), including for assessing resistance to certain treatment options.
In this study, the 42-patient population was enrolled from 5 sites in Canada and 1 site in Scotland, and data were gathered from March 7, 2019, to January 12, 2022; all had interstitial lung disease (ILD) and early-stage non–small cell lung cancer (NSCLC).
Investigators were searching for answers to the question, “Does it matter if a vein- or artery-first approach is used when performing a robotic lobectomy for non-small cell lung cancer (NSCLC)?
This most recent analysis from the CheckMate 9LA trial provides 4-year follow-up data on patients with stage IV metastatic/recurrent non–small cell lung cancer (NSCLC) randomized to treatment with nivolumab plus ipilimumab and chemotherapy vs chemotherapy alone.

The application is based on results of the phase 3 TROPION-Lung01 trial of datopotamab deruxtecan in nonsquamous non–small cell lung cancer (NSCLC), first presented at the 2023 European Society for Medical Oncology Congress.

Besides the new approval, AstraZeneca released preliminary results for LAURA evaluating osimertinib after chemoradiotherapy.

Coverage of Tennessee Oncology's Johann Brandes, MD, on "Precision Medicine in NSCLC."
Consolidation durvalumab led to comparable outcomes among older patients with unresectable stage III non–small cell lung cancer (NSCLC) vs a younger cohort with the same cancer; this patient population was underrepresented in the PACIFIC trial.

This retrospective analysis compared molecular testing outcomes among newly diagnosed cases of metastatic non–small cell lung cancer (mNSCLC) from 2 periods: March 2017 to May 2019 and July 2019 to March 2021.
Outcomes were evaluated among veterans treated for non–small cell lung cancer (NSCLC) from 2017 to 2020 with durvalumab, a PD-L1 inhibitor.

The TROP-2–directed antibody drug conjugate is currently being investigated as a monotherapy and a combination therapy for non–small cell lung cancer (NSCLC) in 3 trials: phase 3 EVOKE-01, phase 2 EVOKE-02, and phase 3 EVOKE-03.

Molly Mendenhall, BSN, RN, director of quality and compliance at Oncology Hematology Care, Inc (OHC), discussed a 1-year quality improvement project implemented by OHC to standardize comprehensive biomarker testing in patients with non–small cell lung cancer (NSCLC).

This new study examined postoperative survival among discharged patients following treatment of non–small cell lung cancer (NSCLC) via surgical resection using the National Cancer Database.