
Rare Disease
Latest News

Latest Videos

CME Content
More News

Sudipto Mukherjee, MD, PhD, MPH, hematology and medical oncology, Cleveland Clinic, suggests that some patients with indolent systemic mastocytosis (ISM) may be progressing at a faster rate than expected, and will need to be monitored and started on appropriate therapies.
Momelotinib was given category 2A and 2B status for patients with high- and low-risk myelofibrosis (MF) and MF with anemia. However, ruxolitinib retains a higher category of recommendation as a treatment for patients with MF.

The most-read rare disease articles include topics on increasing research and awareness on genetic and nongenetic rare diseases.
A recent study found that uptake of disease-modifying therapies (DMTs) has been low among patients with sickle cell disease, suggesting that more interventions that consider individual patient characteristics are needed to improve adoption.
Posters presented at the American Society of Hematology Annual Meeting and Exposition identified factors associated with reducing risk and improving survival in patients with polycythemia vera (PV).

David Fajgenbaum, MD, MBA, MSc, cofounder of Every Cure, shared that the organization is able to reduce clinical trial costs to $1 to $5 million per drug trial, and that clinical trials are not always necessary to expand a drug's indication.

BCMA-directed CAR T-cell therapy anitocabtagene autoleucel demonstrated a 76% complete remission rate and 89% negative minimal residual disease in patients with relapsed/refractory multiple myeloma.
Two posters set to be presented at the 65th American Society of Hematology Annual Meeting & Exposition met their primary and secondary end points regarding exagamglogene autotemcel therapy for sickle cell disease and β-thalassemia.

This data may bring patients with the rare immunobullous disease closer to an eventual FDA-approved treatment.

Ruben A. Mesa, MD, president and executive director of Atrium Health Levine Cancer Institute and Atrium Health Wake Forest Baptist Comprehensive Cancer Center, discusses how value-based care teams can collaborate to ensure diversity and inclusion in clinical trials.

Jeffrey Sippel, MD, MPH, addresses the increasing denial of insurance claims for non-invasive ventilators (NIV) in Medicare Advantage plans, particularly impacting ALS patients, emphasizing the time crunch on patients, and criticizing the financial focus over patients' well-being.

Geri Landman, MD, MPH, cofounder and chair of Moonshots for Unicorns, talks about how the lack of government funding for PGAP3 research led her to take it upon herself to work toward a cure for her daughter.
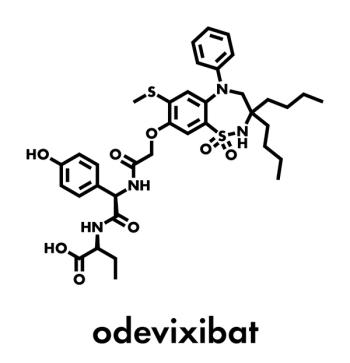
Pooled phase 3 data presented at North American Society for Pediatric Gastroenterology, Hepatology & Nutrition 2023 support the benefit-risk profile of the ileal bile acid transport inhibitor in treating the rare liver disease.

Patient input and experiences play a crucial role in advancing rare disease research and therapy development, as they help define the disease, inform clinical trial design, and influence regulators and payers' decisions, ultimately serving as catalysts for innovation in the field.

Researchers highlighted the potential long-term consequences of COVID-19 on incident autoimmune and autoinflammatory connective tissue disorders in a recent study.

Children of all ages with achondroplasia are now included in the expanded Indication, the most common form of skeletal dysplasia leading to disproportionate short stature.

Of the 333 patients with psoriatic arthritis (PsA) and 457 patients with axial spondyloarthritis (axSpA), there were 1.09 serious infections per 100 patient-years.

This group of cancers remains a challenge to diagnose, can be rapidly progressive, and requires complex treatment and follow-up care, research suggests.

Crinetics announced findings from the PATHFNDR-1 trial showing the investigative oral daily drug may provide patients with the rare disease an alternative from recurring injection therapy.

Nedosiran (Rivfloza) was approved for children and adults with primary hyperoxaluria type 1 (PH1), according to drugmaker Novo Nordisk.

One meta-analysis featuring 8 studies showed that more than half of patients reported a 100% reduction in their generalized tonic-clonic seizures or tonic-clonic seizures.
Several trial participants experienced concerning hepatobiliary events.

A year of research emphasizes the significance of collaboration, standardized approaches, and targeted therapies in rare pulmonary diseases.
Despite conventional guidelines, Mohs micrographic surgery (MMS) may be more effective than wide local excision (WLE) for treatment of localized tumors.

While the challenges in affordability and accessibility emphasize the importance of early awareness and interventions, equitable access to the screening itself poses a crucial hindrance.















