Rare Disease
Latest News

Latest Videos

More News

While the challenges in affordability and accessibility emphasize the importance of early awareness and interventions, equitable access to the screening itself poses a crucial hindrance.

Catherine Nester, RN, vice president of physician and patient strategies at Inozyme Pharma, highlights the importance of newborn screening for rare diseases and the impact of the BeginNGS diagnostic tool.


This rare adverse effect was witnessed in a small number of people post vaccine administration.

Condition-specific support is needed among patients living with rare disease and their family members.

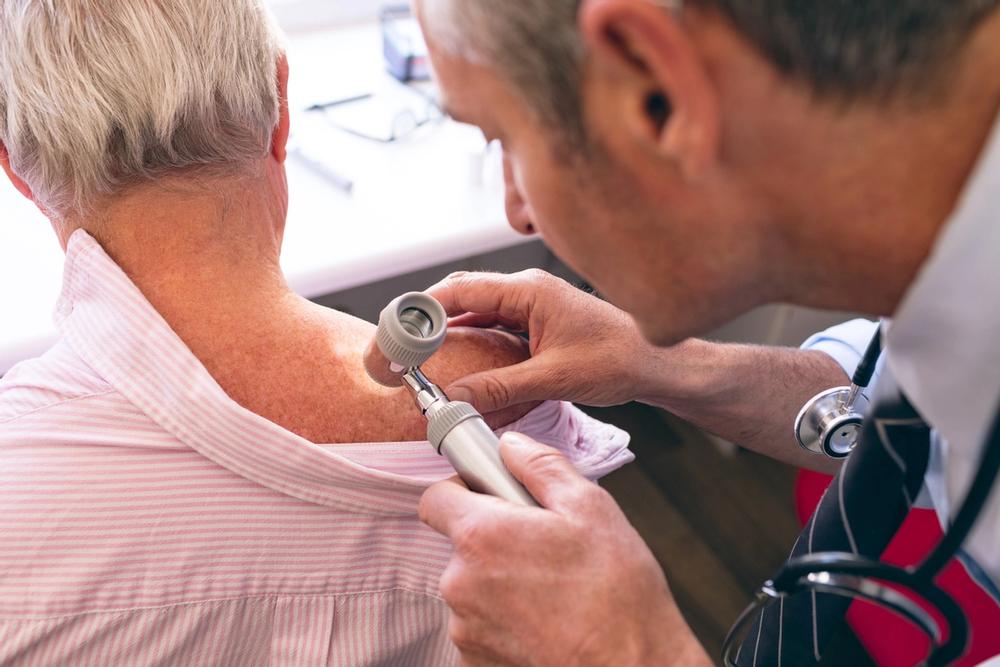
The rare disease can cause burning, stabbing, or shooting pain in patients.

Patients with Prader-Willi syndrome showed early signs of microvascular disease upon screening.
Many health care providers are not aware of alpha-gal syndrome, an allergic condition with a wide range of symptoms, a study suggests.

The greatest reduction in skeletal muscle mass was in the lower extremities, a new report shows.

While antibiotic treatment is highly effective, soft rick relapsing fever (STRF) can result in severe health complications, including death, if not treated in a timely manner.

The retrospective study of patient hospital data showed that current approaches to identifying a culprit drug often overidentify drugs unlikely to be responsible for Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) while potentially missing the actual culprit.

Franco Locatelli, MD, PhD, head of the Department of Pediatric Hematology and Oncology at IRCCS Bambino Gesu Children’s Hospital in Rome, discusses the treatment of both adult and pediatric patients with chronic graft versus host disease (cGvHD).

An Iowa abortion ban is temporarily blocked; Pfizer partners with Flagship Pioneering in drug development deal; South Dakota’s governor calls for action in addressing the national drug shortage.

Franco Locatelli, MD, PhD, a professor and hematologist from Italy, discusses results from the the phase 2 REACH 5 studies assessing the use of ruxolitinib in pediatric patients with chronic graft versus host disease (cGvHD).

More than 4 in 10 participants received a rare disease diagnosis as a result of the analysis.

There is no approved treatment for chronic hepatitis D virus infection in the United States, but recent phase 3 trial results showed encouraging safety and efficacy associated with bulevirtide.

Martin Griesshammer, MD, PhD, Johannes Wesling University Clinic, discusses past and current efforts to improve treatment options for patients with polycythemia vera.

The act was passed in 1983 and has contributed to increased research and development on treatments for rare diseases.

The Mayo Clinic’s Program for Rare and Undiagnosed Diseases was first implemented in 2018.
Since 1930, there have been fewer than 1000 cases of the disease reported around the world.
The advent of gene therapies, which can target specific variants, means pinpointing the genetic roots of each patient’s disease has taken on new importance, researchers said.

Misdiagnosis was common in these patients, and mean time to diagnosis was around 5 years.

Patients with the rare disease typically present with hypertrophic cardiomyopathy and muscular hypotonia before the age of 1.
Treatment with the first-in-class direct and competitive telomerase inhibitor imetelstat resulted in statistically significant and clinically meaningful efficacy in patients with heavily transfusion dependent, non-del(5q) lower-risk myelodysplastic syndrome that is relapsed or refractory to erythropoiesis stimulating agents, according to findings from the phase 3 IMerge trial.

The decreasing cost of DNA sequencing has contributed to an uptick in genomic sequencing for precision diagnostics in rare diseases.