Results bring more competition to treat this genetically driven target for cardiovascular risk.

Results bring more competition to treat this genetically driven target for cardiovascular risk.

New discoveries in atherosclerosis pathways have shed light on the active mechanisms in other diseases such as chronic kidney disease (CKD) and HIV, paving the way for furture therapeutics.

The FINEARTS-HF trial offered novel clinical insights as one of the few cardiovascular trials to feature such a great proportion of female participants.
The SARAH trial was limited to high-risk patients, which the lead investigator said prevented unnecessary exposure to adverse events in patients less at risk of cardiomyopathy.
Follow-up results for the FINEARTS-HF trial focused on specific results for women and men, hyperkalemia risk, and an analysis of the elements of its composite end point.

Lead study author Alexander T. Sandhu, MD, MS, of Stanford said the team will evaluate behavior to understand the results.

A decision from the American Board of Medical Specialties will be made about a new cardiovascular board by this upcoming February.

The connection between brain, mental, and cardiovascular health needs considerable more attention, argues Maureen Hood, PhD, RN, Uniformed Services University.

Posters presented at the American Heart Association Scientific Sessions included results that show how persistent disparities are in cardiovascular (CV) health.

Payers and employers have tightened access to the glucagon-like peptide 1 receptor agonist class once these therapies became more commonly prescribed for obesity.

Rebekah Walker, PhD, a first-time participant from the Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo, shares the offerings and opportunities at the 2024 American Heart Association (AHA) meeting that excited her and her team.

Therapies for obesity and inhibitors for lipoprotein(a) (Lp[a]) are among those to be featured at the American Heart Association (AHA) Scientific Sessions November 16-18, 2024, which will take place in Chicago, where the AHA was founded 100 years ago.

A panel including several experts in cardiology discussed the ways that heart failure (HF) can be diagnosed and treated using the new 2022 AHA/ACC/HFSA Guidelines.

Tochi M. Okwuosa, DO, cardiologist and director of cardio-oncology at Rush University Medical Center, delivered several presentations at the American Heart Association’s Scientific Sessions this year. Chief among them were the importance of cardiovascular health in cancer survivors and cardio-toxicity from cancer treatments.

Kausik K. Ray, MB ChB, MD, MPhil, is professor of public health and a consultant cardiologist at Imperial College London in the United Kingdom. At this year’s American Heart Association’s Scientific Sessions, he presented findings from a 4-year open-label extension study of inclisiran, a small interfering RNA that targets proprotein convertase subtilisin kexin type 9 to lower low-density lipoprotein cholesterol (LDL-C).

Keith C. Ferdinand, MD, FACC, FAHA, FASPC, FNLA, professor of medicine and the Gerald S. Berenson Endowed Chair in Preventative Cardiology, Tulane University School of Medicine, discusses the results of the recently halted FRESH trial, why there is such a great need for new antihypertensive agents, and possible contributory factors to outcome disparities between Black and White patients.

Posters presented at the American Heart Association's Scientific Sessions elaborated on the results of out-of-pocket expenses and adherence for guideline-directed medical therapies in patients with heart failure with reduced ejection fraction (HFrEF).

The DELIVER trial is the largest trial to date of SGLT2 inhibitor use in heart failure, and these latest data on dapagliflozin in heart failure with mildly reduced or preserved ejection fraction show an extensive benefit on health status, noted Mikhail Kosiborod, MD, cardiologist at St. Luke's Mid America Heart Institute in Kansas City, Missouri.

A panel at the annual American Heart Association conference held in Chicago, Illinois, discussed ways in which cardiovascular disease (CVD) care was affected by equity issues between White and Black patients.

The latest real-world clinical practice data from the VICTORIA trial of vericiguat bolster previous data on the medication’s benefit by showing that 92% of patients hospitalized for a worsening heart failure event would be eligible to start the therapy and that doing so would reduce their risk of heart failure hospitalization and cardiovascular death, noted Stephen J. Greene, MD, Duke University Medical Center and the Duke Clinical Research Institute.
Posters presented at the American Heart Association conference in Chicago, Illinois, evaluated the insights from the VICTORIA trial and their generalizability to patients hospitalized with heart failure with reduced ejection fraction (HFrEF).

In the ENNOBLE-ATE trial, Michael A. Portman, MD, FAHA, director, Pediatric Cardiovascular Research, Center for Integrative Brain Research, and professor of pediatrics at Seattle Children's, and his team evaluated the safety and efficacy of edoxaban, a direct oral anticoagulant previously only used among adult patients, among pediatric patients with cardiac disease.

Douglas L. Mann, MD, professor of medicine at Washington University School of Medicine in St. Louis and editor-in-chief of JACC: Basic to Translational Science discussed the first set of data reported on NTLA-2001, a novel investigative intravenous agent that targets the TTR gene and TTR protein levels, which have been shown to play a role in development of cardiac transthyretin (ATTR) amyloidosis.

Research presented at the American Heart Association (AHA) Scientific Sessions in Chicago, Illinois, found that the Kansas City Cardiomyopathy Questionnaire-12 (KCCQ-12) was able to assess patients’ symptoms more accurately for clinicians.

Posters presented at the American Heart Association (AHA) Scientific Sessions in Chicago, Illinois, found that the neighborhood income and socioeconomic status had an effect on heart failure and all-cause readmission rates.
The American Heart Association (AHA) will hold its annual conference in person in Chicago and online from November 5-7, with sessions on health equity and gene editing receiving particular focus.
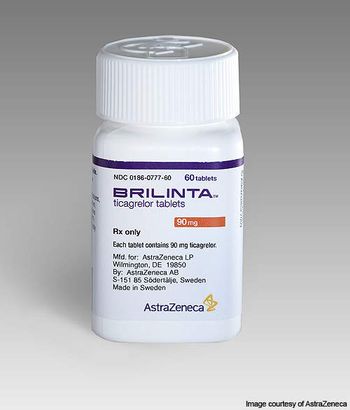
Bleeding is a risk with all antiplatelet drugs, whose effects pose a problem when patients need surgery or suffer a traumatic injury. A fast-acting reversal agent would make the drug safer to use.

Deepak L. Bhatt, MD, MPH, executive director of interventional cardiovascular programs at Brigham and Women’s Hospital Heart & Vascular Center and professor of medicine at Harvard Medical School, explains the interim findings of the REVERSE-IT trial that were presented at the 2021 AHA Scientific Sessions.

A panel Monday at the 2021 American Heart Association Scientific Sessions featured new results for several heart failure (HF) therapeutics, including finerenone and empagliflozin, as well as cost-effectiveness data for vericiguat.

The VICTORIA study found vericiguat to be more cost-effective than placebo when using current societal benchmarks for health care value in the United States. Derek Chew, MD, now an assistant professor at the University of Calgary, conducted economic evaluations for the VICTORIA trial while with the Duke Clinical Research Institute. Results were presented at the 2021 AHA Scientific Sessions. Here, he explains why vericiguat is more cost-effective for patients with heart failure with reduced ejection fraction.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
