
Early and lifelong trauma can intensify menopause symptoms and accelerate cardiovascular and brain aging, according to research.

Early and lifelong trauma can intensify menopause symptoms and accelerate cardiovascular and brain aging, according to research.

Preclinical data suggest a possible synergistic interaction between estrogen and GLP-1 signaling, explains Regina Castaneda, MD.

The AMCP Nexus 2025 conference will bring together professionals to discuss drug access and policy, as well as the future of pharmacy.

US safety data show that elinzanetant caused few adverse events and no new liver-related concerns in women, James Simon, MD, explains.

Hormonal shifts drive fat redistribution, muscle loss, and bone decline—all risk factors for cardiovascular disease in women, says Brooke Aggarwal, EdD.

Treating vasomotor symptoms, sleep disturbance, and mood disorders can remove barriers to weight loss for women during menopause, experts say.
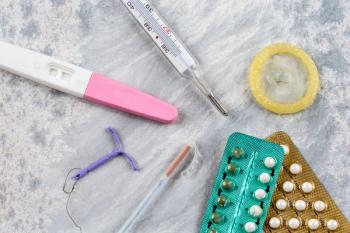
Which hormonal contraceptives are still safe, when should women stop using them, and what other benefits do they offer during the menopause transition?

Elinzanetant offers a promising alternative to hormone therapy for women seeking relief from menopause symptoms, said JoAnn V. Pinkerton, MD.

Susan Escudier, MD, FACP, of Texas Oncology, suggests actionable insights to improve equitable access to patient cancer care

Different cultural, linguistic, and systemic barriers could impact how Indigenous and Hispanic women access menopause care, said Lisa Taylor-Swanson, PhD.

Can women feel relief from tinnitus and burning mouth syndrome during perimenopause and menopause?

A study reveals promising results for combining enfortumab vedotin and pembrolizumab in treating recurrent head and neck cancer, showing a 39% response rate.

Susan Cantrell, CEO of the Academy of Managed Care Pharmacy, shares exclusive insights on key themes that will be discussed at this year's Nexus conference.

Dedicated COPD respiratory therapists can improve inhaler use, ensure adherence, and bridge care transitions, explained Megan Dulohery Scrodin, MD.
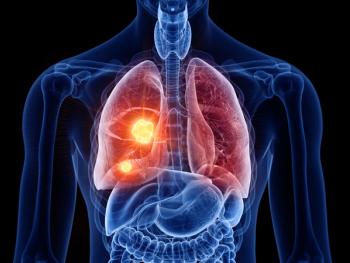
Meta-analyses presented at the CHEST 2025 Annual Meeting find that combinations that include amivantamab and nivolumab improved overall and progression-free survival in non–small cell lung cancer (NSCLC).

Assessing all available nonhormonal and hormonal therapies for vasomotor symptoms helps empower women to make informed decisions, said Lee Shulman, MD.

Digitized behavioral cough suppression therapy is a safe, highly effective, and accessible alternative to drugs for chronic cough, said Laurie Slovarp, PhD.

The efficacy of GLP-1 inhibitors in weight loss has introduced them as a means of also addressing obstructive sleep apnea in sleep medicine.

Experts also explained how hormone therapy can benefit women managing chronic conditions such as diabetes and hypertension on top of their menopause.
A session held during the CHEST 2025 Annual Meeting focused on the North American guidelines for treating bronchiectasis.

CT scans can play a role not just in diagnosing but also in monitoring non–cystic fibrosis bronchiectasis management, explained James D. Chalmers, MD.

IO Biotech's immune-modulatory cancer vaccine combination shows promise in advanced melanoma, despite missing statistical significance for PFS.

Researchers will present new findings on how menopause affects cardiovascular, brain, metabolic, and digestive health.
Perioperative enfortumab vedotin plus pembrolizumab demonstrated significant results in a population that represents roughly half of all patients with MIBC, explained Christof Vulsteke, MD, PhD.

Inhaled corticosteroids pose long-term risks in COPD management, prompting careful screening and potential de-escalation strategies, said Sara Assaf, MD.

Counseling patients with obstructive sleep apnea on using GLP-1s effectively prior to starting treatment can help ensure adherence, said Matthew Biszewski, PharmD.

Balazs Halmos, MD, of Montefiore Albert Einstein Cancer Center, discussed the potential impact of subcutaneous amivantamab EGFR-mutated NSCLC.
New findings reveal that combining Pluvicto with standard therapies significantly improves outcomes for patients with prostate cancer, enhancing quality of life.

Experts at the European Society for Medical Oncology Congress meeting in Berlin, Germany, explore rising cancer rates in young adults, focusing on survivorship challenges and the role of lifestyle interventions in care.

Despite advances, Arielle Kauvar, MD, calls for more research on laser and energy-based devices for patients with skin of color.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
