Researchers determined that migraineurs exhibit unique responses to tactile stimuli compared with controls, according to a study published in Cephalalgia.

Researchers determined that migraineurs exhibit unique responses to tactile stimuli compared with controls, according to a study published in Cephalalgia.

According to recent study results published in JAMA, treatment-limiting Physician Orders for Life-Sustaining Treatment (POLSTs) were significantly associated with lower rates of intensive care unit admissions among patients with life-limiting conditions compared with patients who had full-treatment POLSTs. However, researchers found 38% of patients with treatment-limiting POLSTs still received intensive care that was potentially discordant with their preferences.
Investigators say that inconsistencies in the way rare diseases are defined contribute to misdiagnoses, delayed treatment, and other ills that could be addressed with global standards.
The 6 finalists will pitch their innovations at Google’s campus February 27 for a chance to win up to $50,000 in in-kind services from Boston Scientific and Google.

A vascular surgeon in Florida allegedly stole $26 million from insurers and the government; a new report finds many rural areas are still at risk of an HIV outbreak; new disposable flavored vape pens have largely replaced Juul use among teens.

Close to 80% of HIV-positive individuals are shown to be virally suppressed through their most recent test results, according to data from 2016 through 2018, as well as 32% to 63% of adults older than 24 years. Youth with a new HIV diagnosis, however, come in at only 12%.

Sickle-cell trait (SCT) and disease (SCD) among African American patients were associated with faster kidney function decline, with SCD contributing to a more rapid decline, according to study findings.
A systematic review and meta-analysis of 10 studies to evaluate the efficacy and safety of pharmacological treatments for lymphangioleiomyomatosis yielded mixed results, according to the findings published in Respiratory Research.
Finely tuned eye movements are instrumental in enhancing visual acuity, according to findings of a study published in Nature Communications.

Statin use was linked with a significant reduction in the risk of hospitalized exacerbations after an initial hospitalized exacerbation among patients with chronic obstructive pulmonary disease (COPD), including specified frequent exacerbators, according to study findings.

The use of sodium-glucose cotransporter 2 (SGLT2) inhibitors leads to cardiovascular benefits in all patients with type 2 diabetes (T2D), according to a study recently published in the Journal of the American Heart Association.

Passengers of the coronavirus-infected ship Diamond Princess landed in the United States Monday, including 14 infected with the virus; 1 in 4 opioid overdoses were shown in a study to involve children 18 years old and younger; World Health Organization (WHO) initiative on insulin prices gains support.
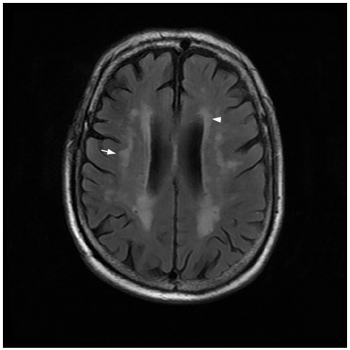
These findings suggest that baseline white matter hyperintensities can act as a predictive marker or therapeutic target for the development of levodopa-induced dyskinesia in patients with early stage Parkinson disease.
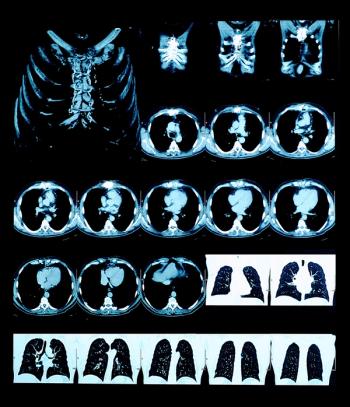
A decision aid delivered through tobacco quitlines may improve informed decision making about lung cancer screening, according to study findings.

There’s not much research available comparing the Paleolithic diet to other popular nutritional plans. A new study sought to scour existing data to see the Paleolithic diet’s effect on glucose and insulin regulation. The data showed no significant impact.
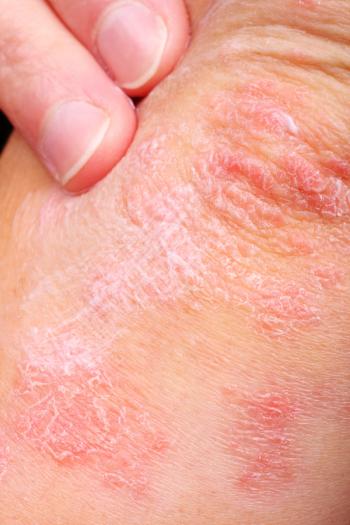
Patients undergoing immunotherapy could be at risk for psoriasis flare-ups, even if the patient is asymptomatic at the initiation of cancer treatment. A newly published case report outlines a rare case where immunotherapy had to be stopped due to severe skin lesions.

Primary and acquired resistance to bortezomib can increase recurrence rates in patients with multiple myeloma. A new strategy could help reduce resistance.

Michael Abrams, MA, is the co-founder and managing partner of Numerof & Associates, a firm that helps businesses across the healthcare industry define and implement strategies for winning in dynamic markets. He has more than 25 years of expertise working helping clients navigate an evolving healthcare landscape, across hospital systems, payers, and Fortune 500 pharmaceutical, device, and diagnostics companies. He has co-authored several books and has been featured in leading business journals and news outlets.

The Narcolepsy Monitor—a companion app for long-term narcolepsy symptom monitoring—may be a helpful tool for gaining insight into the individual burden of narcolepsy symptoms over time and may serve as a patient-reported outcome measure, according to study findings.

The 2007 paper examined the elevated risks for cardiovascular disease and type 2 diabetes for patients who have schizophrenia or bipolar disorder.

Results showed that managing T1D becomes more intense during pregnancy and in the months that follow childbirth. This is due to the need to be even more vigilant than usual in monitoring blood glucose levels and dosing insulin, tasks the women performed mostly themselves, but which can also be performed by other healthcare professionals.

Flow cytometry (FCM), next-generation sequencing, (NGS), and polymerase chain reaction (PCR) are the 3 most common methods clinicians use to diagnose minimal residual disease (MRD) in patients with acute lymphoblastic leukemia who have undergone chemotherapy, radiotherapy, or immunotherapy. MRD can be found both in bone marrow via aspiration and peripheral blood circulation through a draw.
The 5-year survival rate for triple-negative breast cancer (TNBC) is about 77%. The recurrence rate is highest in the first 3 years after treatment, but falls off at the 5-year mark—although the survival rate at this time point tends to be lower. Because TNBC cells lack the hormone receptors for estrogen and progesterone and do not overexpress the human epidermal growth factor receptor 2 gene, treatment often involves chemotherapy, radiation, and surgery. Targeted treatments are not used with TNBC.
New research confirms that end-stage renal disease is a significant risk factor for patients undergoing cataract surgery.
Through a series of live-cell single-molecule imaging experiments, researchers at the University of York discovered how rogue communications between cells lead to leukemia, according to a recent study published in Science.

Writing for the panel, Judge David Sentelle said HHS Secretary Alex Azar ignored predictions that thousands of people would lose their healthcare coverage.

Coverage of our peer-reviewed research and news reporting in the healthcare and mainstream press.

A federal watchdog will take on Medicare scams; Belviq, a weight loss drug, has been pulled from market; issues at Veterans Affairs have resulted in healthcare roadblocks.

This week, top managed care stories included CareMore Health successfully managing complex patients; HHS standing by its rules on interoperability; healthcare costs rising faster than wages.
Interstitial lung disease requires a multidisciplinary clinical approach, though little is known about effective therapeutic treatments.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
