
Making value-based payment models work in cancer care requires close attention to the financial and quality measures and a commitment to culture change, said experts gathered October 25 at the Community Oncology Alliance Payer Exchange Summit.
Mary Caffrey is the Executive Editor for The American Journal of Managed Care® (AJMC®). She joined AJMC® in 2013 and is the primary staff editor for Evidence-Based Oncology, the multistakeholder publication that reaches 22,000+ oncology providers, policy makers and formulary decision makers. She is also part of the team that oversees speaker recruitment and panel preparations for AJMC®'s premier annual oncology meeting, Patient-Centered Oncology Care®. For more than a decade, Mary has covered ASCO, ASH, ACC and other leading scientific meetings for AJMC readers.
Mary has a BA in communications and philosophy from Loyola University New Orleans. You can connect with Mary on LinkedIn.

Making value-based payment models work in cancer care requires close attention to the financial and quality measures and a commitment to culture change, said experts gathered October 25 at the Community Oncology Alliance Payer Exchange Summit.

Panelists who have reviewed the Enhancing Oncology Model (EOM) say it puts additional requirements on practices with reduced monthly payments, and its risk modeling could put a small practice out of business.


Selected coverage from the Quality Cancer Care Alliance (QCCA) Summer 2022 National Leadership Summit, the National Comprehensive Cancer Network (NCCN) Fall Policy Summit, and the American Association for Cancer Research (AACR) Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved.

A featured presentation at the Irvine, California, meeting of the Institute for Value-Based Medicine discussed how a patient with long-term HIV was cured through a transplant.

Coverage from the Irvine, California, meeting of the Institute for Value-Based Medicine, chaired by Joseph Alvarnas, MD, vice president for government affairs at City of Hope and chief clinical advisor, AccessHope.

Coverage from the Minnesota meeting of the Institute for Value-Based Medicine, chaired by Rajini Katipamula-Malisetti, MD, vice president of medical oncology at Minnesota Oncology.

Although exercise during cancer treatment is encouraged, multiple myeloma can present special challenges, since there is an increased risk of bone fractures, pain, and other deformities.

Leaders from City of Hope National Medical Center discussed how the cancer research and treatment center addressed challenges with attracting and retaining oncology pharmacists through a restructuring during the ACCC 39th National Oncology Conference.

Mike Koroscik, vice president, Oncology, Allina Health Cancer Institute, presented “Preparing for Population Health in Oncology,” during the Association of Community Cancer Centers 39th National Oncology Conference, which concluded Friday.

Winners of the Association of Community Cancer Centers 2022 Innovator Awards, presented at the National Oncology Conference, include a program to embed primary care in a cancer center and an effort to increase compliance with giving patients same-day medication education.

Ochsner Health's program was among the recipients of the 2022 ACCC Innovator Awards, given to projects that improve patient care, are cost-effective, and are replicable.

The sessions align with the yearlong theme selected by this year’s ACCC president, David R. Penberthy, MD, MBA, when he took the helm in March: “Leveraging Technology to Transform Cancer Care Delivery and the Patient Experience.”
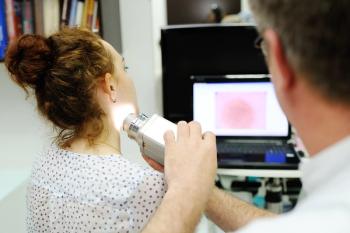
Dermoscopy is a relatively inexpensive and readily available tool that can improve outcomes and quality of life for patients who need surgical excision of nonmelanoma skin cancers, the authors found.

Along with its position statement in Diabetologia, the group outlined recommendations that seek greater structure and consistency for automated insulin delivery (AID) manufacturers as they develop products for use by persons with type 1 and type 2 diabetes.

Regular assessments of patient-reported outcomes (PROs) are important to deliver the best clinical care, including the monitoring health-related quality of life (HRQOL), researchers reported.

Co-hosted by Memorial Sloan Kettering Cancer Center, the most recent Institute for Value-Based Medicine® event took place on September 22, with a focus on improving cancer care delivery through innovation.

William Oh, MD, chief medical officer at Sema4 and a clinical professor of medicine at Mount Sinai, discusses Prostate Cancer Awareness Month and possible reasons for disparities in diagnoses and mortality in prostate cancer.

The annual report notes that progress in reducing cancer mortality is uneven among populations, with minority groups not seeing the same benefits from therapeutic advances. Cancers related to obesity are also on the rise.

Danielle Carnival, PhD, addressed the National Comprehensive Cancer Network Policy Summit on Friday.

The National Comprehensive Cancer Network recommendations come as the FDA weighs an indication in chronic lymphocytic leukemia/small lymphocytic leukemia (CLL/SLL) for zanubrutinib.

For several years, investigators have examined the potential for allogenic natural killer (NK) cells as an alternative for “off-the-shelf” chimeric antigen receptor (CAR) treatments. New study results presented at the International Myeloma Society (IMS) meeting showed CD38 CAR-NK cells significantly reduced the tumor burden—and improved survival
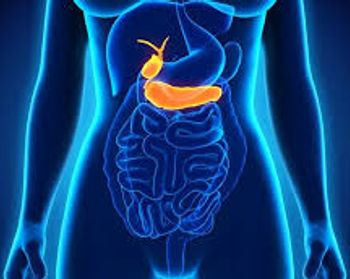
An estimated 1 in 4 patients treated with durvalumab and chemotherapy (gemcitabine plus cisplatin) was alive at 2 years compared with 1 in 10 treated with chemotherapy alone, with these results contributing to the FDA's approval of the first immunotherapy to treat these cancers.
Patients receiving the quadruplet therapy have continued to show improved responses and higher rates of minimal residual disease (MRD) negativity.

Katherine R. Tuttle, MD, FASN, FACP, FNKF, a nephrologist from the University of Washington and Providence Health Care, discussed new consensus guidelines that call for the early use of sodium glucose cotransporter 2 (SGLT2) inhibitors, GLP-1 receptor agonists, and finerenone in the care of patients with both chronic kidney disease (CKD) and diabetes.




Ide-cel is a B-cell maturation antigen (BCMA)-directed CAR T-cell therapy, which uses the process of genetically modifying a patient’s T cells and infusing them back into the patient to attack the cancer.

New heart failure guidelines redefine stages of the disease to emphasize prevention, said Biykem Bozkurt, MD, PhD, but more must be done by payers to identify those at high risk.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
