
A series of experiments identified both "good" and "bad" gut bacteria, suggesting possible treatments for obesity.
Mary Caffrey is the Executive Editor for The American Journal of Managed Care® (AJMC®). She joined AJMC® in 2013 and is the primary staff editor for Evidence-Based Oncology, the multistakeholder publication that reaches 22,000+ oncology providers, policy makers and formulary decision makers. She is also part of the team that oversees speaker recruitment and panel preparations for AJMC®'s premier annual oncology meeting, Patient-Centered Oncology Care®. For more than a decade, Mary has covered ASCO, ASH, ACC and other leading scientific meetings for AJMC readers.
Mary has a BA in communications and philosophy from Loyola University New Orleans. You can connect with Mary on LinkedIn.

A series of experiments identified both "good" and "bad" gut bacteria, suggesting possible treatments for obesity.
The bill is part of President Donald Trump's blueprint to rein in prescription drug prices, and advocates see it as a small step in the right direction.
Lower socioeconomic status is associated with poor survival in lymphoma.

The study by the National Institute on Aging produced a few surprises and was stopped early when aspirin showed no benefit.
The announcement comes with word from FDA touting the effectiveness of its efforts to advance digital health regulatory pathways.
The application is based on results presented in March at the 67th Scientific Session of the American College of Cardiology.
One patient saw a complete response, and the therapy was well tolerated, especially compared with the adverse effects sometimes seen with chimeric antigen receptor (CAR) T-cell treatment in blood cancers thus far.
FDA's designation is based on phase 2 data presented earlier this year at the American Society of Clinical Oncology Annual Meeting.
The analysis of patient records found no elevated risk of retinopathy among those using glucagon-like peptide-1 (GLP-1) receptor agonists to treat type 2 diabetes.

A leader at the Institute for Healthcare Improvement offers a framework for diabetes educators to embrace a shift in thinking about healthcare delivery.



Authors of a Health Affairs blog post cite an initiative that rates how well cities are doing in making sure their laws allow for affordable housing, which is getting more attention as a factor in population health.

Patients with type 2 diabetes and moderate renal impairment who took oral semaglutide in a phase 3a trial reported a larger reduction in glycated hemoglobin and more weight loss than patients taking placebo.

Three federal officials discussed the status of research, payer coverage, and referrals for the National Diabetes Prevention Program, as well as the rollout of the Medicare program for eligible seniors.

The rise of digital health alongside the transformation of reimbursement from fee-for-service to value-based care is allowing patients to fully participate in their own care, according to a leader with one of the top digital health companies in diabetes care.

Robert A. Gabbay, MD, PhD, FACP, chief medical officer and senior vice president at Joslin Diabetes Center, said health systems need people with the skill sets that diabetes educators possess to make the transition to a reimbursement system based on quality, prevention, and eliminating costs.

Thea Danze lights up the room wherever she goes; she loves space and music and fire trucks. Since 2012, she has been the namesake and spokesperson for a foundation created to raise funds and awareness about pediatric brain tumors. Called Thea’s Star of Hope, the foundation supports development of therapies that will treat brain tumors without the toxic adverse effects that Thea has endured.

FAA policy on granting medical certificates to people who use insulin are based on policies created in 1949, and advocates for people with diabetes say there's been a revolution in technology and medication since then.
LifeScan, the diabetes division that Johnson & Johnson shed after ending US sales of its Animas insulin pump, will be the sole preferred provider of meters and testing strips for Express Scripts members in 2019.

States that ranked poorly are those with high rates of diabetes and obesity; those with high rankings have long-term commitments to getting people insured.
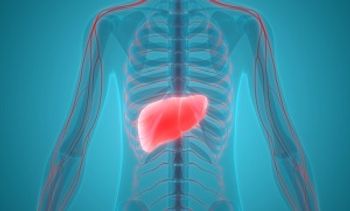
The lead author of a study appearing this month in Diabetes Care said other data support his findings, pointing to the potential for the SGLT2 inhibitor empagliflozin to replace metformin as first-line therapy.

While overall cardiac deaths have dropped in recent decades, sudden cardiac deaths have not seen a similar decrease. Of the 200,000 people who have a sudden cardiac arrest in the hospital, only 24% survive.
Eli Lilly this week announced it has submitted applications to US and European regulatory agencies for nasal glucagon, which, if approved, could shave minutes and stress from the current method for delivering this hormone to people with diabetes experiencing severe hypoglycemia.


Findings from the Foundation for Government Accountability did not net out those who would be eligible for exemptions under CMS rules.
While the connection between diabetes and cancer has been known, this study was the first to show that women with diabetes face a higher risk than men with the disease.

Sulfonylureas are an older class of type 2 diabetes therapy but remain the most commonly prescribed antidiabetic drug after metformin.

Findings were consistent with earlier studies that link long work hours and diabetes as well as long periods of sitting with diabetes and obesity.

Interest groups representing drug companies, patients, providers, and health plans all submitted comments to a Request for Information on the Trump administration blueprint for controlling drug prices and out-of-pocket costs.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
