
Earlier this week, the US Preventive Services Task Force (USPSTF) released a draft guidance recommendations on medications for women at an increased risk for breast cancer.

Earlier this week, the US Preventive Services Task Force (USPSTF) released a draft guidance recommendations on medications for women at an increased risk for breast cancer.
While treatment for HIV has made tremendous strides over the past few decades, many patients with the infection still struggle with comorbidities such as chronic inflammation, diabetes, and cardiovascular problems, among others. In a recent study, researchers at Michigan State University sought to understand why patients with HIV develop such complications.

Researchers hypothesized that because coffee and caffeine showed a beneficial effect on daytime tiredness in Parkinson disease, a similar positive effect might be assumed in multiple sclerosis (MS). Investigators compiled a systematic review focused on summarizing the possible effects of coffee and caffeine in MS.
A recent prospective phase 2 study sought to investigate autologous hematopoietic stem cell transplantation as a therapeutic intervention in multiple sclerosis (MS).



A recent study looked to evaluate the real-world effectiveness of hepatitis C virus (HCV) treatment in patients with genotype 1 (HCV-1) infection.
A recent trial looked to compare the efficacy and safety of pembrolizumab (Keytruda) with the standard of care for the treatment of head and neck squamous cell carcinoma (SCC).
The efficacy and safety of glecaprevir/pibrentasvir (G/P, sold as Mavyret) in the treatment of hepatitis C virus (HCV) has previously only been investigated in clinical trials. Thus far, no real-world data had been available until a group of researchers looked to investigate the efficacy and safety of G/P in a real-world setting in Italy.

Earlier this week, the FDA granted accelerated approval to pembrolizumab (Keytruda) for adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma (MCC).
Hepatitis C virus (HCV) is the most frequently reported bloodborne infection in the United States, and prevalence has increased in recent years. Researchers recently sought to estimate the prevalence of HCV at the state level in order to more accurately guide prevention and care efforts.

In 2018, The American Journal of Managed Care® team traveled all over the country to provide conference coverage for some of the biggest meetings in the industry. Here are the top 5 most-read conference pieces from this year.

Existing data are limited on the treatment of chronic hepatitis C virus (HCV) in patients with cancer. In a study recently published in Nature, researchers sought to evaluate the safety and efficacy of a sofosbuvir (Sovaldi)-based therapy in this patient population.
During the San Antonio Breast Cancer Symposium held in San Antonio, Texas from December 4-8, 2018, Biotheranostics presented new data evaluating the impact of the Breast Cancer Index (BCI) in patients with early stage hormone receptor positive (HR+) breast cancer who are classified as an immediate risk for distant recurrence by previous genomic testing.


In November, AstraZeneca announced that the phase 3 MYSTIC trial investigating durvalumab (Imfinzi) monotherapy and durvalumab in combination with tremelimumab in patients with previously untreated stage IV non–small cell lung cancer (NSCLC) did not meet its primary end point.
BioNTech AG, a drug developer that specializes in cancer immunotherapies, announced last month new results from a clinical performance evaluation study of a molecular in vitro diagnostic test for quantitative detection of mRNA expression of certain genes.

Recently, 6 European organizations appealed a European Patent Office (EPO) decision to uphold Gilead Science’s patent on the hepatitis C drug sofosbuvir, sold as Sovaldi.

December 2-8, 2018, marks the annual National Influenza Vaccination Week. Here are 5 things to know about getting your flu shot.
A recent retrospective review of patients who contracted hepatitis C (HCV) in childhood found that those with perinatal infection developed cirrhosis earlier than other risk groups.

In a study presented at the annual meeting of the Radiological Society of North America (RSNA), researchers found that starting mammography screening at age 30 may benefit women with at least 1 of 3 risk factors: dense breasts, a personal history of breast cancer, or a family history of breast cancer.
This week, the FDA approved larotrectinib, to be sold as Vitrakvi, for the treatment of adult and pediatric patients with solid tumors that have a neurotrophic receptor tyrosine kinase (NTRK) gene infusion without a known acquired resistance mutation, are metastatic or where surgical resection is likely to result in severe morbidity, and have no satisfactory alternative treatments or that have progressed following treatment.
A recent study found that a combination treatment of glecaprevir and pibrentasvir is both effective and well tolerated in patients with chronic hepatitis C virus (HCV) who have failed previous therapies.
Earlier this month, Merck announced that the FDA approved its anti–PD-1 therapy, pembrolizumab (sold as Keytruda), for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib.
A recent study sought to learn more about how estrogen receptor signaling occurs in vivo in normal and cancerous tissue.
A new study found that in 50% of patients, the standard 12-week treatment regimen for hepatitis C could be shortened to as few as 6 weeks without compromising efficacy.
Researchers from Massachusetts General Hospital and the Broad institute of MIT and Harvard recently identified specific states of cytotoxic CD8+ T cells that are associated with patient response to checkpoint immunotherapy for melanoma.
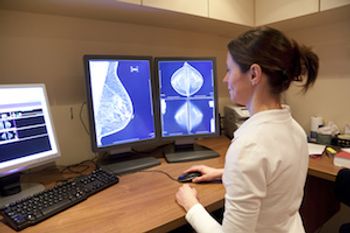
Researchers recently reported findings from a study designed to determine patient attitudes about mammographic reporting of breast arterial calcification (BAC), result of the BAC communication, and subsequent action.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
