
At least 8 biosimilars were approved in Canada over the past 12 months, as policy makers made a strong push for biosimilar acceptance, which led to a change in prescribing patterns.

At least 8 biosimilars were approved in Canada over the past 12 months, as policy makers made a strong push for biosimilar acceptance, which led to a change in prescribing patterns.

In hopes of protecting its lucrative adalimumab (Humira) franchise, AbbVie is mounting a hefty legal defense against Alvotech's adalimumab biosimilar candidate.

Investigators said pathologic complete response was higher with a molecularly selected subgroup of patients with early-stage breast cancer treated with a nonchemotherapy combination that included a trastuzumab biosimilar.

Recent surveys shed light on oncologist and patient perceptions of the switch to biosimilars and patient mental health.
Costs of extending human epidermal growth factor receptor 2 (HER2) therapy for patients with metastatic breast cancer were estimated in a retrospective study.

Investigators hope to duplicate promising phase 2b trial results with an experimental peptide vaccine in a population of nearly 500 patients with human epidermal growth factor receptor 2 breast cancer.
The list of potential adalimumab biosimilars has now lengthened to 10, according to a Cardinal Health summary of these products and their distinguishing features.

Generics and biosimilars are reimbursed differently, causing providers to prefer lower-cost generics and higher-priced biologics, authors of a study and opinion piece contend.

The European Medicines Agency (EMA) reports on progress to create a smoother, less wasteful biosimilar development process; and the World Health Organization (WHO) revises its biosimilar development guidelines.
Sandoz, Lupin, and Biocon Biologics representatives at the Association for Accessible Medicines (AAM) GRx+Biosims conference said that drug price rebates hinder biosimilar competition.
For trastuzumab and bevacizumab, biosimilars now represent a high share of administrations, but payer policies still hinder uptake of these products, the Community Oncology Alliance (COA) reports.

Genetic profiling in recurrent and advanced breast cancer can yield actionable, smoking-gun biomarkers, Stanford Cancer Institute pathologists explained at the National Comprehensive Cancer Network 2021 Virtual Congress: Biomarkers in Solid Tumors.

There is now a mad scramble to gain interchangeable status for biosimilars, but the meaning and significance of this appellation haven't yet been worked out for health care consumers or the manufacturing community.

An overview of activities at the FDA to promote the use of biosimilars was presented by Jacqueline Corrigan-Curay, JD, MD, principal deputy center director for the Center for Drug Evaluation and Research (CDER) at the FDA.
Evidence is insubstantial for treatment of Clostridioides difficile infection (CDI), but authors of a review of available evidence provide recommendations for agents and testing.
Pharmacy experts from IPD Analytics explained the potential for authorized biologics, which would compete, potentially on the same price footing, with biosimilars.
In studies presented at the American College of Gastroenterology 2021 meeting, success was reported for RBX2660, a treatment for clostridioides difficile infection (CDI).

Education is needed for both patients and pharmacists to ensure appropriate understanding, investigators reported at the Academy of Managed Care Pharmacy (AMCP) Nexus 2021 meeting.
Studies presented at Academy of Managed Care Pharmacy Nexus 2021 elucidated costs of skin treatment for workers in terms of recovery time taken and percentage of salary.
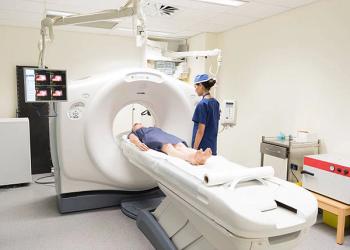
For patients with diffuse large B-cell lymphoma (DLBCL), PET/CT scans conducted in the first cycle of salvage therapy can predict for response, according to a study.

A review of treatment options in diffuse large B-cell lymphoma (DLBCL) addresses common subtypes and patient response to standard of care.

The designation means that pharmacists can switch patients to the less-expensive version of insulin without seeking approval from the clinician.
Bispecific T-cell engagers and chimeric antigen receptor T-cell therapies seem destined to move into earlier lines of therapy for multiple myeloma (MM), speakers at the European Hematology Association 2021 Virtual Congress said.

Using limited data from the ASPEN trial, investigators estimated survival probability and cost-effectiveness for zanubrutinib vs other agents in Waldenström macroglobulinemia.

There are many unknowns for third-line chronic myeloid leukemia (CML) treatment and beyond, speakers said at the EHA2021 Virtual Congress.

For Alberta and Green Shield Canada, savings are beginning to add up from switching initiatives for multiple reference biologics.

Hidradenitis suppurativa (HS) is a difficult-to-treat skin condition that has responded to infliximab, although payer support in this setting is often lacking.

Hospital reimbursement deals slow the adoption of biosimilars in oncology, but cost concerns contribute to the incentives to use these agents.

The challenges of complying with payer mandates in biosimilars were discussed in a webinar sponsored by The American Journal of Managed Care® and The Center for Biosimilars®.

Patients who research medical information online are likely to be more concerned about switching from originator products to biosimilars, according to the study.

Published: December 15th 2021 | Updated:

Published: December 13th 2021 | Updated:

Published: April 22nd 2021 | Updated:

Published: August 29th 2021 | Updated:

Published: May 2nd 2018 | Updated:

Published: March 28th 2020 | Updated:

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
