Fabry Disease
Latest News
Latest Videos

CME Content
More News
A recent review highlighted the importance of managing cardiovascular risk factors in patients with Fabry disease versus the general population.
The parameter could potentially help clinicians zero in on the cause of left ventricular hypertrophy.
New data show the therapy led to minimal changes in renal function, even after more than 8 years.

Even compared with other patients with chronic diseases, patients with Fabry disease had a very poor quality of life.
Early diagnosis of Fabry disease is essential and that since symptoms depend on the type of disease and sex and age of the patient, a high-risk screening system should account for the age of the target population, the researchers said.

Most patients with Fabry disease reported decreased physical and mental health-related quality of life (HRQOL) over 13 years of follow-up.

Pain is a hallmark of many Fabry disease cases, and improvements in management could dramatically improve quality of life for patients.
A small study, based on 4 family members with Fabry disease, affirms the importance of early enzyme replacement therapy.
About 1 in 3 patients experienced adverse drug reactions in the 2 new safety studies, aligning with earlier reports.
All-cause graft failure in kidney transplant recipients with Fabry disease was about 30%, and improved when enzyme replacement therapy became available.

Despite concerns that patients with Fabry disease might be at heightened risk of severe symptoms from SARS-CoV-2, early data find that most patients experience mild or moderate symptoms.

These national and regional barriers, which range from cost of screening to patient education, have hindered the widespread use of screening, say the researchers.
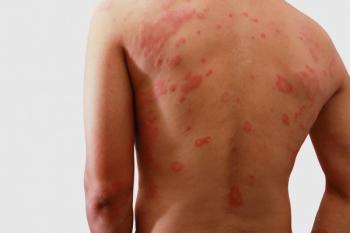
This is a case study of a male patient with Fabry disease who presented with severe anaphylaxis following re-initiation of full-strength treatment with intravenous agalsidase beta (Fabrazyme).

The Patient Access Network (PAN) Foundation has opened a new patient assistance program for people living with Fabry disease/

Biotechnology company Avrobio has completed a $60 million Series B financing to advance multiple gene therapies, including AVR-RD-01, a proposed single-dose lentiviral gene therapy for Fabry disease (FD).