Oncology
Latest News
Latest Videos

CME Content
More News

CAR T-Cell therapy updates presented at the American Society of Clinical Oncology's Annual Meeting held June 2018.

AJMC®TV interviews let you catch up on what’s new and important about changes in healthcare, with insights from key decision makers—from the clinician, to the health plan leader, to the regulator. When every minute in your day matters, AJMC®TV interviews keep you informed. Access the video clips at ajmc.com/interviews.



Care strategies updates from the American Society of Clinical Oncology's Annual Meeting, held June 2018.












In both primary care and specialty practices, nurse practitioners not only have a growing presence, but they also play an increasingly key role in delivering care to patients in these settings.
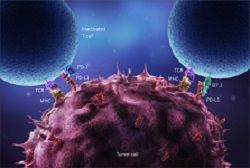
KEYNOTE-048, a phase 3 trial studying pembrolizumab (Keytruda) as a first-line treatment for patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) whose tumors overexpress PD-L1, has met a primary end point of overall survival.

Clinical findings as presented at the American Society of Clinical Oncology's Annual Meeting in June 2018.

We know that there are a lot of problems with precision medicine and lots of areas that we need to define better, but that shouldn’t stop us from moving forward and trying to optimize the care for our patients, said Michael Thompson, MD, PhD, FASCO, Aurora Advanced Healthcare.

During the 2018 American Society of Clinical Oncology’s (ASCO) Annual Meeting, researchers presented findings on 3 biosimilar trastuzumab products: Samsung Bioepis’ SB3, Agmen’s ABP 980, and Biocad’s Herticad.

A second biosimilar to Amgen’s Neupogen, which treats neutropenia, has been approved in the United States. The FDA approved Pfizer’s filgrastim-aafi, which will be sold under the brand name Nivestym. Zarxio (filgrastim-sndz) was the first filgrastim biosimilar approved by the FDA in 2015.
A study of over 35,000 women with early-stage breast cancer found that where you live makes a difference in terms of what kind of posttreatment imaging is received, due to geographic variation.