
The patient had hemophilia A, but reported no trauma preceding the hematoma.

The patient had hemophilia A, but reported no trauma preceding the hematoma.

The results of a new focus group offer insights on how and when people with rare diseases are willing to share their personal health information.
Iptacopan elicited improved hemoglobin levels measuring at least 2 g/dL higher vs baseline, leading to transfusion independence after 24 weeks in approximately 92.2% of patients with complement inhibitor–naïve paroxysmal nocturnal hemoglobinuria.

Gene therapies could have a major impact, although the cost and complexities could inhibit their wide rollout, study authors said.

Investigators found patients with acute coronary syndrome were 3 times more likely to die in the hospital.
The data highlight that silent cerebral infarcts represent just a portion of brain injury that occurs in patients with sickle cell disease (SCD), and brain volume can serve as another potential biomarker of brain injury in those with the disease.

These findings underscore the need for improved bleed protection and hemophilia care for those with all severities of the disease.

For all the success the hematology/oncology space has seen over the past 20-plus years, difficult discussions now focus on paying for that care, explained David A. Eagle, MD, New York Cancer & Blood Specialists.

CMS in February 2023 announced 3 new models for testing by the Center for Medicare & Medicaid Innovation—all of which “aim to lower the cost of drugs, promote accessibility to life-changing drug therapies, and improve quality of care.”
A review of cases involving Jehovah’s Witnesses found many modalities can be successfully deployed to treat thrombotic thrombocytopenic purpura (TTP).

The findings suggest recombinant ADAMTS-13 may be a useful adjunct therapy to plasma exchange among patients who have thrombotic thrombocytopenic purpura (iTTP).

The patient’s thrombotic thrombocytopenic purpura (TTP)–like symptoms were caused by deficiencies of vitamins B1 and B12.
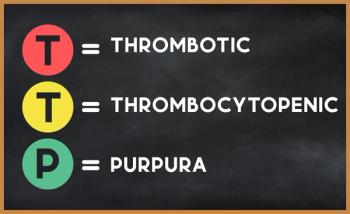
The impaired reactivity may play a role in organ injury, the authors suggested, among patients who have immune-mediated thrombotic thrombocytopenic purpura (iTTP).

Judicious use of caplacizumab (Cablivi) can reduce the risk of severe bleeding and help manage costs associated with the therapy in patients living with thrombotic thrombocytopenic purpura (TTP).

New data underscore the long-term implications of immune-mediated thrombotic thrombocytopenic purpura (iTTP) and atypical hemolytic-uremic syndrome (aHUS), both rare diseases.

People with the inherited disease can expect to live 52.6 years following birth, the report shows.

A substantial proportion of families of privately insured children with sickle cell anemia pay more than $100 for essential stroke screenings, a high-value service.
Investigators said the Mortality in TTP Score (MITS) was effective at characterizing the risk of death in patients hospitalized with thrombotic thrombocytopenic purpura (TTP).

Investigators said several new anemia therapies are in development, and many of them may help wide swaths of patients.

Efanesoctocog alfa is the first factor VIII therapy to overcome the interaction with endogenous von Willebrand factor, which creates a ceiling of 8 to 19 hours on the half-life of current factor VIII replacement products, which in turn creates the need for more frequent dosing.

The reality of a sickle cell disease cure brings fear, optimism, and questions to patients’ lives; veterans who are in suicidal crisis can receive free emergency care at any Department of Veterans Affairs or private facility; Pfizer to increase access to progressive treatments through sale of drugs at non-profit prices to poor countries.

The most-read rare blood articles included studies on cold agglutinin disease , hemophilia B gene therapy, autoimmune hemolytic anemia, and hereditary thrombotic thrombocytopenia purpura.

A pair of abstracts presented during this year’s 64th American Society of Hematology Annual Meeting and Exposition explored barriers to treatment for sickle cell disease (SCD).
A pair of abstracts presented at this year’s 64th American Society of Hematology Annual Meeting and Exposition bear out the significant transfusion-free rate at 3 years following beti-cell administration and marked improvements in patient-reported outcomes, including the ability to work and be physically active.
Mental and physical health aspects of quality of life (QOL) improved after sutimlimab treatment in patients with cold agglutin disease (CAD) during a phase 3 trial.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
