
Two posters presented at Kidney Week 2023 evaluated the global state of chronic kidney disease (CKD) and shed light on delays in CKD diagnoses in the United States.

Two posters presented at Kidney Week 2023 evaluated the global state of chronic kidney disease (CKD) and shed light on delays in CKD diagnoses in the United States.
Presenters at the American Society of Nephrology's Kidney Week 2023 discussed the past, present, and future of pediatric chronic kidney disease, as well as explored the obstacles and solutions for effective trial designs.

Coverage of our peer-reviewed research and news reporting in the health care and mainstream press.
Postoperative circulating tumor DNA (ctDNA)–based minimal residual disease status demonstrated prognostic value for patients with stage 1 to 4 resected colorectal cancer, showing that those with positive ctDNA after surgery have significantly lower disease-free survival outcomes at 24 months vs those who were ctDNA negative.

A study evaluating disparities among children with acute leukemia found that Black children are more likely to present with higher disease burden at diagnosis compared with non-Hispanic White children.

Researchers highlighted the potential long-term consequences of COVID-19 on incident autoimmune and autoinflammatory connective tissue disorders in a recent study.
The Medical Group Management Association offers an analysis of pending legislation that could help health care practices better serve patients.

Sickle cell disease (SCD) has known complications that include organ damage and failure, rhabdomyolysis, glaucoma, splenic sequestration, and vaso-occlusive crises. At present, the only potential cure is a bone marrow transplant, but it can be difficult to find a donor and there is a high rejection risk.
While response and safety may vary across racial and ethnic subgroups, progression-free survival and overall survival does not appear to differ when chimeric antigen receptor T-cell therapy is used in the treatment of patients with multiple myeloma.

Two posters presented at the 2023 Fall Clinical Dermatology conference identified common patterns among patients with psoriasis (PsO) and psoriatic arthritis (PsA).

Despite e-cigarette use dropping 4.1% in the past year, it is still the most commonly used tobacco product among both middle and high school students.
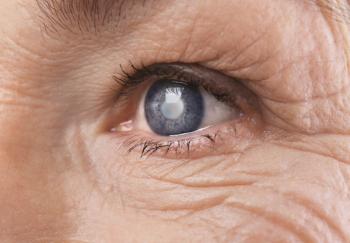
Medicare beneficiaries in California in 2019 had multiple causes of glaucoma and primary open-angle glaucoma, indicating a need to investigate undiagnosed glaucoma.

The new vaccine program will protect against influenza and COVID-19.
High glucose variability in children and young adults with type 1 diabetes (T1D) strongly predicted slowed nerve conduction velocity, a forerunner of diabetic peripheral neuropathy, in a recent study.
The drug’s mode of action is different than other types of corticosteroids because it’s based on the differential effects on glucocorticoid and mineralocorticoid receptors.

The American Cancer Society expanded eligibility for lung cancer screening; experts advised patients to do their research before choosing a plan from the Affordable Care Act’s insurance marketplaces; Republican-led states partnering with rideshare companies for medical appointment rides.
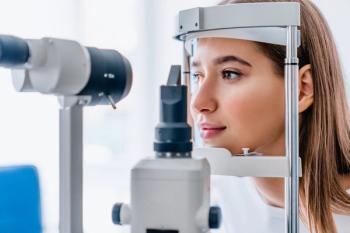
Toku’s platform aims to deliver real-time cardiovascular disease (CVD) risk assessments using routine eye exams.
After implementing a multidisciplinary surgical approach, researchers found that use of the new approach, residual disease, and age were all independent predictors of overall and progression-free survival for patients with ovarian cancer.
Immune thrombotic thrombocytopenic purpura (TTP) can be treated safely and effectively with caplacizumab.

In a study driven by simulation modeling, researchers elaborated on policies that contribute to widening health disparities affecting Indigenous communities.

The findings from a recent study unveiled notable distinctions in bleeding rates between etranacogene dezaparvovec and the standard prophylactic factor IX (FIX) products among individuals diagnosed with hemophilia B.

Wezlana is the first biosimilar to reference Stelara approved in the United States. The product will launch in 2025.

Men who had sex with men had increased HIV testing after participating in an intervention.

A panel of experts agreed that exa-cel, a sickle cell disease gene therapy, was safe enough for clinical use, setting the stage for a potential FDA approval; the United States saw an increase in infant mortality rates for the first time in more than 2 decades; the new antiburnout campaign from the CDC asks leaders to better support health care workers.

Patients with colorectal cancer (CRC) can use C-reactive protein (CRP), lymphocyte to CRP ratio, and CRP to albumin ratio as markers for CRC prognosis.

The approval, which is based on results from the phase 3 KEYNOTE-966 trial, marks the sixth indication for pembrolizumab in gastrointestinal cancer.
The addition of durvalumab to first-line chemotherapy, followed by maintenance treatment with durvalumab plus olaparib significantly improved progression-free survival in patients with newly diagnosed advanced or recurrent endometrial cancer, according to data from the phase 3 DUO-E/GOG-3041/ENGOT-EN10 trial.
Central nervous system (CNS) progression was reduced with osimertinib plus chemotherapy for patients with EGFR-mutated non-small cell lung cancer plus CNS metastases.

Investigators evaluated the utility of the MG Symptoms Patient-Reported Outcome (PRO) within the armamentarium of instruments that currently evaluate myasthenia gravis (MG) severity.

The approval of secukinumab is the second approved biologic to treat hidradenitis suppurativa.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
