The model that incorporated environmental and lifestyle data collected from a fitness tracker and a smartphone app was successful at predicting acute exacerbation of chronic obstructive pulmonary disease (AECOPD) 7 days in advance.

The model that incorporated environmental and lifestyle data collected from a fitness tracker and a smartphone app was successful at predicting acute exacerbation of chronic obstructive pulmonary disease (AECOPD) 7 days in advance.

On this episode of Managed Care Cast, Patricia Salber, MD, MBA, of The Doctor Weighs In, talks with the founder and CEO of ConsejoSano, a patient engagement firm working with diverse, multicultural, multilingual populations about the importance of building relationships and trust when trying to understand opinions and beliefs about COVID-19 vaccination.

The 7-day average of new COVID-19 cases in India reaches a record high; vaccination rates of US adults differ significantly depending on the region; 1 million people have enrolled during the special ACA open enrollment period.

Laura Crotty Alexander, MD, ATSF, outlines her presentation on e-cigarette or vaping product use-associated lung injury (EVALI) and other vaping diseases in the time of COVID-19 and beyond.

The FDA said vaccinating adolescents is another step in getting the country back to prepandemic life.

A report using data from the DAPA-CKD study found that dapagliflozin would lead to more than $99 million in savings in a population matched to the trial’s inclusion criteria.
An observational study reveals COVID-19 social distancing measures affected migraineurs' sleep, physical activity.
A roundup of cancer news from the ESMO Breast Cancer Virtual Congress 2021, which took place last week and was covered by OncLive®.

Dupilumab is effective for atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyps, but a recent presentation noted the payer challenge in placing the biologic in an optimal tier.

The FDA cleared Bigfoot BioMedical's Diabetes Management System.
A small study, based on 4 family members with Fabry disease, affirms the importance of early enzyme replacement therapy.

HHS announces prohibitions against discriminating by sexual orientation, gender identity in health care; updated CDC guidance highlights airborne threat of COVID-19; European Union, Pfizer/BioNTech agree to deal that could reach 1.8 billion vaccine doses.
Frank Martin, PhD, director of research at JDRF, shares feedback from patients on JDRF's T1Detect screening program.

Michelle M Cloutier, MD, is professor emerita at UCONN Health in Farmington, Connecticut, and chair of the National Asthma Education and Prevention Program (NAEPP) Coordinating Committee Expert Panel Working Group.

As the COVID-19 pandemic has laid bare issues of rising high health care costs and disproportionate coverage nationwide, executive decision makers at large employers indicated their support for greater government intervention to address concerns.

Soft tissue and bone infections, urinary tract infections, stroke, and electrolyte disorder top the reasons why patients with diabetes are admitted to the hospital at greater frequency and cost compared with patients without the disease, according to a new study.
Strategies for improving outcomes across hematologic cancers and solid tumors range from addressing cytokine release syndrome and neurotoxicity mediators, immune rejection, on-target off-tumor toxicity, post-infusion control limitation, and immunosuppressive tumor microenvironment.
BK virus–specific T cells from healthy donors were an effective and safe off-the-shelf treatment for patients with leukemia or lymphoma who developed a painful and common complication after allogeneic hematopoietic stem cell transplantation.

Biomarkers are being used in patients with cancer to target treatments that will work best. Interviews with OncLive® highlight how biomarkers are being used in colorectal and bladder cancer.
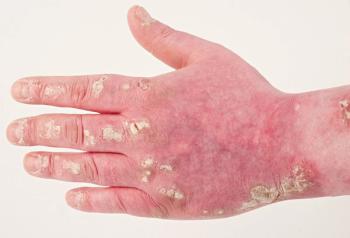
Using acitretin in combination with secukinumab resulted in nearly complete or total relief for 3 patients with severe psoriasis, according to a case study.

Intravitreal dexamethasone implants stabilized, but did not improve, vision for most patients with macular edema due to retinal vein occlusion (RVO).

A roundup of diabetes news you may have missed from across MJH Life Sciences™.

Although knowledge is plentiful on disease state improvements for adult patients with multiple sclerosis (MS) following use of newer disease-modifying therapies, less is known about similar outcomes among patients with pediatric-onset MS (POMS).
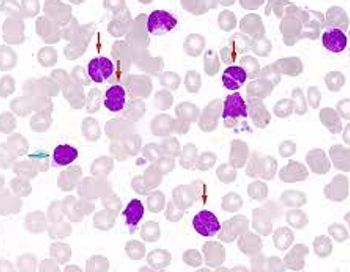
Through matching-adjusted indirect comparison, the authors of the abstract compared the use of umbralisib with other PI3Kis in this setting and found that the treatment yielded the lowest discontinuation rates due to adverse events (AEs), had a lower AE management cost burden, and achieved better duration of responses.

Specific factors, such as experience with technology and health literacy, were key drivers of whether a patient with chronic obstructive pulmonary disease (COPD) would benefit or be likely to use eHealth tools to improve self-management skills, investigators concluded.

About 90% of patients are diagnosed with rheumatoid arthritis in an outpatient setting, while the 10% diagnosed in an emergency department or as inpatients incur significantly higher health care costs.
William Shrank, MD, MSHS, the chief medical officer of Humana, discusses how the company is trying to overcome any vaccine hesitancy both in its workforce and in its insured member population.
The literature review included 25 studies related to diagnostic impact of liquid biopsies versus tissue biopsies and 3 studies that assessed the economic impacts of the 2 biopsies.
In a first-of-its kind study, young children undergoing heart surgery had similar results regardless of whether they received levosimendan intravenously or via inhalation.

The group-based Sleep Apnea Management clinic at Cleveland Clinic's Sleep Disorders Center increased criteria-met adherence to positive airway pressure (PAP) therapy by 20% in patients with obstructive sleep apnea (OSA).

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
