
Preliminary data released by CMS show black Americans with Medicare coverage are nearly 4 times more likely than their white counterparts to be hospitalized for coronavirus disease 2019 (COVID-19).

Preliminary data released by CMS show black Americans with Medicare coverage are nearly 4 times more likely than their white counterparts to be hospitalized for coronavirus disease 2019 (COVID-19).
Acute kidney injury is a sudden onset of kidney damage that causes waste products to build up in the body. These episodes have become more common during the coronavirus disease 2019 pandemic, causing kidney failure in some previously healthy patients who had no prior history of renal problems.

Authors reviewed the current landscape of therapeutic interventions for differentiating pain classifications in patients with Parkinson disease, noting the need for further research in pathophysiology-driven treatment.

Researchers find that 80% of cases of coronavirus disease 2019 (COVID-19) may have gone undiagnosed in March; Sanofi Pasteur expands partnership to speed up vaccine development; FDA issues warning on 9 hand sanitizers produced by Mexico-based manufacturer.

Inflammatory skin diseases are often diagnosed by "first impression" and can be easily misdiagnosed. A new smartphone app using artificial intelligence (AI) may be able to assist with the diagnosis of psoriasis, atopic dermatitis, and eczema.
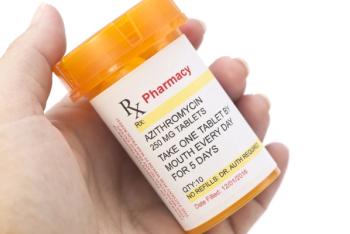
Compared with amoxicillin, azithromycin was shown in a recent study to increase both the relative and absolute risks of cardiovascular death among patients being treated on an outpatient basis.

Currently, retinoblastoma (RB) occurs in 1 of every 20,000 births, making it the most common pediatric intraocular neoplasm. The cancer results from biallelic inactivation of the RB1 tumor-suppressor gene, which encodes the nuclear phosphoprotein RB protein.
In a review, researchers outlined the safety and efficacy of erenumab, the only monoclonal antibody built to interact with the calcitonin gene related peptide (CGRP) receptor rather than the CGRP itself, currently indicated as a preventive treatment for migraine.

An online program designed specifically for adolescents and young adults who survived cancer was shown to significantly alleviate insomnia and improve overall quality of life, according to study findings published today.
Among Medicare enrollees, there was substantial between-practice variation in the use of second-generation diabetes drugs between 2007 and 2015, according to a study published in JAMA Network Open. Data also revealed a concentration of use among a few prescribers and practices, who were responsible for widespread early diffusion.

Public health experts and presidentail staffers express frustration over coronavirus disease 2019 (COVID-19) testing comments made by President Trump; the NIH has halted a clinical trial of hydroxychloroquine after data show no harm or benefit in patients with COVID-19; individuals who have obsessive-compulsive disorder may be at greater risk due to added stress from the pandemic.

The study authors tested the risk-reducing effects of ocrelizumab compared with interferon β-1a for treatment of multiple sclerosis disability accumulation, with ocrelizumab showing better results in lowering disability accumulation scores.

Inhaled corticosteroids for patients with chronic obstructive pulmonary disease (COPD) may curb lung function decline, according to new research.
The impact of psoriatic arthritis on quality of life can vary depending on geographic location and culture, according to a global patient survey.

Central nervous system gene therapies and advanced technologies to provide these treatments were noted as emerging opportunities in treatment for neurological conditions such as Parkinson disease, according to review findings. Researchers also highlighted the lack of experience and technology in medical centers nationwide to provide these services.

In a poster titled “Basal Insulins in Advanced Renal Failure: Time for a Paradigm Shift,” authors compared the safety and efficacy of insulin degludec (Tresiba) with insulin glargine 100U (Lantus) among patients with stage 3 and stage 4 chronic kidney disease (CKD).
Most studies of patients with diffuse large B-cell lymphoma do not include those over the age of 80. However, a new study says those patients, like younger patients, benefit from receiving rituximab and anthracycline in their chemotherapy regimens when possible.

A look at the Diabetes Prevention Program at the 10-year mark showed the program brought lasting results.

Regular physical exercise reduces the risk of developing age-related cataracts (ARC), according to a dose-response meta-analysis published in the International Journal of Ophthalmology. ARC is one of the leading causes of vision impairment throughout the world and is estimated to account for 13.4 million cases of blindness.
A new case report highlights the potential links between certain acute cardiac issues and the early stages of multiple sclerosis.
Two studies evaluated how well the dual inhibitor reduced glycated hemoglobin (A1C) while measuring instances of hypoglycemia and other safety measures.
Regardless of smoking status, chronic obstructive pulmonary disease (COPD) is an independent risk factor for developing lung, colorectal, liver and other cancers in the Korean population, according to a study published in BMC Pulmonary Medicine.

In 90% of patients, psoriasis precedes psoriatic arthritis by an average of 7 years, providing a window of opportunity for early intervention and possible prevention of psoriatic arthritis.

Homeless individuals are more likely to be put on ventilators for respiratory infections than non-homeless individuals, according to research published in the Journal of General Internal Medicine.

There is a positive association between having at least 1 chronic disease and adhering to guidelines for breast cancer screening, according to recently published study results.

Coverage of our peer-reviewed research and news reporting in the healthcare and mainstream press.

Diagnosis pediatric acute lymphoblastic leukemia (ALL) can be a costly and cumbersome experience. New research has identified microRNA signatures that could simplify the process of diagnosis and classifying the cancer.

The director of the National Cancer Institute said models predict excess cancer deaths in the thousands due to delayed care and screening; Democratic senators call for an investigation into for-profit institutional review boards; US hospitals begin administering dexamethasone to the sickest patients.

This week, the top managed care news included more details about the COVID-19 vaccine process; attempts to reinforce social distancing amid reopening; a recap of the American Diabetes Association 2020 Virtual Scientific Sessions.

Based on the safety and efficacy outcomes from the PREVENT phase 3 study, the FDA has approved secukinumab (Cosentyx) from Novartis for the treatment of active nonradiographic axial spondyloarthritis.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
