
An executive with Jazz Pharmaceuticals said the ability to extend survival time in patients with secondary AML, and potentially offer them improved odds for transplant is an advance over traditional chemotherapy.
Mary Caffrey is the Executive Editor for The American Journal of Managed Care® (AJMC®). She joined AJMC® in 2013 and is the primary staff editor for Evidence-Based Oncology, the multistakeholder publication that reaches 22,000+ oncology providers, policy makers and formulary decision makers. She is also part of the team that oversees speaker recruitment and panel preparations for AJMC®'s premier annual oncology meeting, Patient-Centered Oncology Care®. For more than a decade, Mary has covered ASCO, ASH, ACC and other leading scientific meetings for AJMC readers.
Mary has a BA in communications and philosophy from Loyola University New Orleans. You can connect with Mary on LinkedIn.

An executive with Jazz Pharmaceuticals said the ability to extend survival time in patients with secondary AML, and potentially offer them improved odds for transplant is an advance over traditional chemotherapy.

Advances in continuous glucose monitoring, reimbursement for genetic testing, and payment models in oncology care were popular with readers of the Evidence-Based series.
A team at The University of Texas MD Anderson Cancer Center in Houston led by original pioneers in immuno-oncology have published a paper in Nature Medicine that discusses an immune-suppressing enzyme that was strongly present in glioblastoma but not in 5 other tumor types the team studied.
The company presented updated phase 1 results for a revamped version of bb2121 that point to sustained responses for patients with relapsed/refractory multiple myeloma.
As more patients with cancer survive, the risk of cardiac complications due to the effects of therapies has become a concern to oncologists and cardiologists alike.

A federal appeals court today struck down the individual mandate—the heart of the Affordable Care Act (ACA) that requires everyone to have health coverage and lays the groundwork for a risk pool that is more balanced between the sick and the healthy, the young and the old.
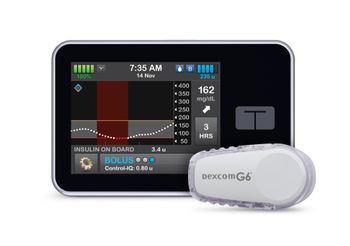
The company said that with the clearance, the FDA outlined standards for a new device category, the interoperable automated glycemic controller designation.

An article Schleicher co-wrote in JAMA Oncology, which appeared the day before the announcement of Oncology Care First, offered insights into the need for changes to the original OCM.

Kashyap Patel, MD, the chief executive officer of Carolina Blood and Cancer Care Associates—a leading OCM practice—and associate editor of Evidence-Based Oncology™ (EBO), said he’s optimistic about Oncology Care First.

The essential role of pediatricians in identifying children who may be at risk for ASD cannot be overstated. This is the first update to the report from the American Academy of Pediatrics since 2007, and it reflects changes in science, the law, and the rise of care coordination.
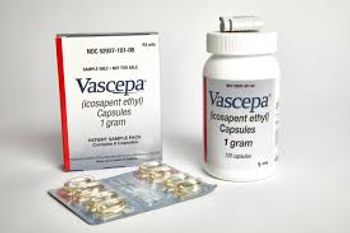
The agency said this was the first such approval, as the purified omega-3 fatty acid is now approved to be used alongside statins to treat elevated cholesterol levels and cut the risk of events such as heart attacks or strokes. The drug was first approved in 2012 for patients with elevated triglycerides.

Governor Henry McMaster, a Republican, announced the waiver plan at an event in Greenville, South Carolina, with CMS Administrator Seema Verma by his side. Some observers see Verma’s promotion of Medicaid work rules in the face of court challenges as a key to her political survival in her feud with HHS Secretary Alex Azar.

The results are sure to generate interest as heart failure with preserved ejection fraction lacks treatment options, but that could change as results are expected in outcomes trials that are studying sodium glucose co-transporter 2 inhibitors in heart failure, both with preserved and reduced ejection fraction.

Although survival rates for multiple myeloma have improved as treatment options have increased, the disease remains incurable, and many patients stop current agents such as lenalidomide or bortezomib due to toxicity. Results of CANDOR involving a triple therapy were presented Tuesday at the 61st American Society of Hematology Annual Meeting and Exposition in Orlando, Florida.

The lead investigator of the study from the Children's Oncology Group said the findings represent a new standard of care.

The study's primary end point, overall survival, showed that patients taking CC-486 had a 31% lower risk of death than those taking placebo.

The French biotech Servier has an agreement with Allogene Therapeutics, through Pfizer, to market its allogenic chimeric antigen receptor (CAR) T-cell product in the United States if it receives FDA approval.

Results presented at ASH support giving ibrutinib as first-line therapy in chronic lymphocytic leukemia (CLL), and future results may offer insights on whether patients can stop therapy once they have undetectable minimal residual disease (MRD).

Results from Avalere Health show that once Medicare patients get through the experience and expense of CAR T-cell therapy, they do well and costs drop significantly.

Successors to the first generation of chimeric antigen receptor (CAR) T-cell treatments will attack multiple targets and address the complexity of the manufacturing process by bringing uniformity to the creation of therapies, presenters said at the 61st American Society of Hematology Annual Meeting and Exposition in Orlando, Florida.

The findings from researchers at The Centers for Disease Control and Prevention (CDC) highlight the public health risks of obesity and type 2 diabetes, which have been tied to recent studies that find rising deaths from heart failure and even an overall drop in US life expectancy, with the long-term rise in obesity playing a role in the decline.

Although the study did not pinpoint the exact mechanism behind the link, the authors wrote that the presence of plaque below the gumline can allow oral bacteria to reach the circulatory system. Certain bacteria that reach the gut can trigger inflammation.
Two recent studies have explored the role of activator protein-1 (AP-1), a transcription factor with a role in skin diseases caused by inflammation, including psoriasis and atopic dermatitis
The findings have important implications, the authors wrote, as the study confirms “the potential clinical utility of empagliflozin for the treatment of obesity and related metabolic disorders, such as insulin resistance, type 2 diabetes, and [nonalcoholic steatohepatitis."

Three straight years of declining life expectancy—from 2014 to 2017—have roots that go back to the 1980s, when the authors say US life expectancy began to lose pace with other countries.
A new study says that more than 1 in 10 of the 17 million cancer survivors in the United States will die from cardiovascular disease (CVD).
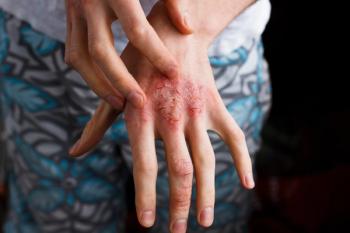
BE READY is designed to evaluate the safety and efficacy of bimekizumab, humanized monoclonal IgG1 antibody that targets and neutralizes interleukin- (IL-)17A and IL-17F, a pair of cytokines that propel the inflammatory process through their effects on other messengers in the body that trigger chronic inflammatory response.

Humana outlined its progress in its third annual Value-Based Care Report, which details growth and evolution in this area since 2016, both in the number of agreements and in its spread across more parts of the country.
EMPRISE (Empagliflozin Comparative Effectiveness and Safety) will examine 5 years of real-world data on empagliflozin, which is sold as Jardiance by Eli Lilly and Boehringer Ingelheim. The study is comparing data on empagliflozin with that for dipeptidyl peptidase 4 (DPP-4) inhibitors. This latest interim analysis also features a cohort comparison with glucagon-like peptide 1 (GLP-1) receptor agonists.

Mavacamten is a first-in-class small-molecule therapy that reduces the contractility of cardiac muscles by binding with myosin, a protein involved in muscle contraction that is often affected by a gene mutation in hypertrophic cardiomyopathy.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
