Breast Cancer
Latest News
Latest Videos

CME Content
More News

Cases of triple-negative breast cancer (TNBC) account for up to 20% of all cases of the disease, the most common cancer diagnosed in women in the United States.

A 20-year follow-up comprising a secondary analysis to an original study shows that high-dose chemotherapy plus hematopoietic stem cell transplant benefit patients with high-risk stage III disease with 10 or more axillary lymph nodes involved.

Previous studies show that up to 75% of women with breast cancer exhibit disease- and treatment-related affects that include poorer cognitive function in the forms of psychological well-being, decision making, and adherence to treatment.

There are more than 50 drug regimens available to treat metastatic breast cancer but little guidance on the best order to deliver them, according to a study published in JCO Clinical Cancer Informatics.

Older age was shown to influence the decision among women to undergo surgery as a treatment option following a diagnosis of breast cancer in the United Kingdom, where the average lifespan was 82.9 years in 2016.

Following decades that saw its popularity dwindle in favor of subpectoral breast reconstruction, prepectoral breast reconstruction is once again on the rise.
In the 2 decades following their diagnosis for ductal carcinoma in situ (DCIS), women in a study out of England demonstrated a greater risk of invasive breast cancer and mortality compared with the general population. Overall, DCIS represents close to 20% of screening-detected breast cancers every year.

A majority of women younger than 45 years faced employment and insurance coverage difficulties following treatment for early stage breast cancer, with 35% fearing loss of health insurance coverage if they left their current job during treatment—despite wanting to continue working.

A small trial out of Columbia University Irving Medical Center shows encouraging recovery results among patients with breast cancer who contracted coronavirus disease 2019 (COVID-19), which was confirmed by reverse transcription-polymerase chain reaction, and/or high clinical or radiographic suspicion. Hospitalization was not necessary for nearly three-quarters of the patients.

Female survivors of breast cancer total over 3.5 million in the United States alone. Coronary heart disease is a leading cause of death among this patient group, as it is for all women.
Fatal breast cancers were reduced by 41% and advanced breast cancers by 25% following recommended screening for the disease by mammography in 9 counties in Sweden, emphasizing the importance of early detection.

Compared with tumors smaller than 8 mm among patients with hormone receptor (HR)-positive, ERBB2-positive (formerly HER2-positive) breast cancer, tumors between 8 and 10 mm benefited more from postoperative chemotherapy.

More than 20% of breast cancer survivors are severely affected by breast cancer–related lymphedema, with debilitating adverse effects that include depression, chronic pain, and recurrent skin infections—all affecting overall quality of life.

Coverage of our peer-reviewed research and news reporting in the healthcare and mainstream press.

In new data from the phase 3 EMBRACA trial presented yesterday at the American Association for Cancer Research annual meeting, researchers found that PARP inhibitor talazoparib exhibited no statistically significant benefit in the secondary end point of overall survival in patients with metastatic HER2-negative breast cancer and mutations in the BRCA1/2 genes.
An international team of investigators recently tried to answer this question, focusing on women with diagnosed early-stage disease and considered obese, with a body mass index above 30 kg/m2.

The FDA granted accelerated approval to Immunomedics’ Trodelvy (sacituzumab govitecan-hziy) as the first antibody-drug conjugate that targets the Trop-2 antigen. Trodelvy is indicated for treatment of relapsed or refractory metastatic triple-negative breast cancer (TNBC) that has spread to other parts of the body.

Cognitive impairment is a well-documented adverse effect of treatment from breast cancer. Complications from it can appear soon after treatment has begun or far down the road.

There is a greater risk of hormone receptor–positive breast cancer if a patient is obese. Researchers from the University of Louisville have discovered a possible new link between obesity and a greater risk for developing breast cancer: adipose fatty acid binding protein.

Several prominent oncology organizations, including the National Comprehensive Cancer Network and the Commission on Cancer, have joined forces to issue preliminary guidelines on how to treat patients with breast cancer during the coronavirus disease 2019 (COVID-19) pandemic.
Meeting HHS’ minimum requirements for daily and weekly exercise reduced breast cancer recurrence and mortality among patients with high-risk breast cancer undergoing chemotherapy. This benefit was even seen in patients who had not met these requirements before their diagnosis.

The standard therapy for triple-negative breast cancer (TNBC) remains chemotherapy, despite a dismal prognosis due to lack of estrogen and progesterone hormone receptors, as well as HER2 receptors. Targeted therapies for this difficult-to-treat, often aggressive, subtype of breast cancer remain elusive.

What can be done to further delineate the risk factors associated with breast cancer to increase prevention efforts across the board? The key may lie in the white blood cells that circulate in the blood, particularly leukocytes and monocytes.

The 2 main criteria that warrant genetic testing for breast cancer in women are age and having a family history of cancer. Postmenopausal women without any hereditary risk factors, however, often do not undergo genetic testing for the disease.
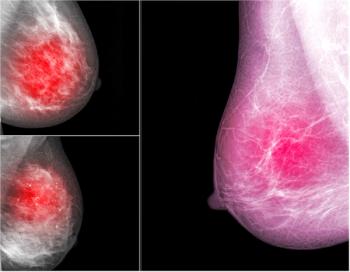
Not all women with dense breast tissue have a high risk of breast cancer, but they all have an increased risk compared with women who have average tissue density. Can this patient population benefit from screening with abbreviated breast magnetic resonance imaging (AB MRI) over digital breast tomosynthesis?