
An innovative CRISPR-Cas12f-based activator system may hold significant potential in the future of gene therapy and biological research, suggest researchers of a new study.

An innovative CRISPR-Cas12f-based activator system may hold significant potential in the future of gene therapy and biological research, suggest researchers of a new study.

The gene therapy giroctocogene fitelparvovec demonstrated superiority compared with the standard of care, routine prophylaxis, in hemophilia A treatment.
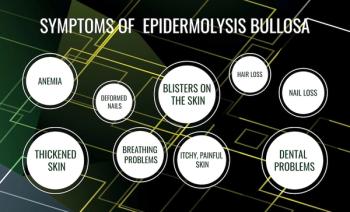
Beremagene geperpavec-svdt (B-VEC) entered the market in 2023 as the first approved corrective treatment for dystrophic epidermolysis bullosa (DEB), a rare genetic disease affecting the skin and nails that is caused by mutations in the COL7A1 gene.

The Greater Philadelphia Business Coalition on Health webinar series provided an in-depth framework for the advantages of including cell and gene therapy coverage in employer health plans, as well as the challenges brought by manufacturing complexities and the need to address accessibility to treatment.
The therapy led to increased frataxin levels and decreased left ventricular mass in patients with Friedreich ataxia (FA) cardiomyopathy, the authors said.
The biomarker, G-protein-coupled receptor 176 (GPR176), may be a driver in the progression of ovarian cancer and potential target for gene therapy, suggested researchers of a new study.

The interim results of a single-arm trial in children with autosomal recessive deafness 9 (DFNB9) show the benefits of binaural administration of gene therapy: better hearing with no serious adverse effects.

An adeno-associated virus (AAV) gene therapy for hereditary spastic paraplegia type 50 (SPG50)—funded by a young patient’s family—gives hope not only to a 4-year-old, but to researchers working on similar projects for other rare diseases.

Early tests show stem cells can be used to spark expression of a miniature version of the dystrophin protein.

Accelerated approval was originally granted in June 2023 for patients aged 4 to 5 years, indicating there was an unmet clinical need for a potentially life-saving treatment for the rare genetic muscle disorder.

The FDA granted the Regenerative Medicine Advanced Therapy (RMAT) designation after reviewing the phase 1 safety and efficacy data.

Building on prior research, a team tackled mouse-model colon cancer with a novel nonviral nanovector to carry dual–messenger RNA (mRNA) immunogene therapy.
The proof-of-concept study showed that antisense oligonucleotides developed by the researchers successfully restored cellular development and brain cell function in patients with the disease characterized by multiorgan dysfunction.

Migvis Monduy, MD, medical director of Neuromuscular and Movement Disorders Programs at Nicklaus Children's Hospital, discussed the most promising areas of research in Duchenne muscular dystrophy (DMD), as well as the mechanisms of gene therapies for DMD.
A new report says early evidence of using gene therapy to treat monogenic diseases offers reason for optimism.

New study findings demonstrate that valoctocogene roxaparvovec, a gene therapy for severe hemophilia A, significantly reduces bleeding episodes and dependency on factor VIII infusions over a 3-year period, while maintaining a favorable safety profile.
This study introduces a novel "all-in-one" strategy combining CD7 chimeric antigen receptor T-cell therapy with haploidentical hematopoietic stem-cell transplantation, showing significant potential to improve survival and reduce toxicities in patients with relapsed or refractory CD7-positive hematologic cancers.

Data from the study showed that exa-cel prevented vaso-occlusive crises in all but 1 of the 30 evaluable patients for at least 12 months.
Only 11 cases of adverse events (AEs) were reported over a 6-year time frame, the investigators found, following administration of chimeric antigen receptor T-cell therapy (CAR T).
The analysis also found the therapy has a manageable safety profile.

In their preclinical model, the researchers found efficacy both in vitro and in vivo by using CRISPR-Cas9 to mimic porphyria and combining the technology with light therapy.

A single injection of a gene therapy was well tolerated and showed the potential to control exudation in patients with neovascular age-related macular degeneration (wet AMD).

The approval makes atidarsagene autotemcel (arsa-cel [Lenmeldy]; Orchard Therapeutics) the first approved treatment for metachromatic leukodystrophy, a disease caused by a mutation in the ARSA gene and marked by progressive declines in both the central and peripheral nervous systems.

In a recent issue of JAMA, a team led by Benjamin N. Rome, MD, MPH, examined how the FDA’s accelerated approval process has moved 5 genetically targeted treatments for Duchenne muscular dystrophy (DMD) through its pipeline despite limited evidence on their efficacy.

Since 2016, 5 targeted treatments have received accelerated approval from the FDA for use in Duchenne muscular dystrophy (DMD), and the total spend for just 3 of them is $3.1 billion.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
