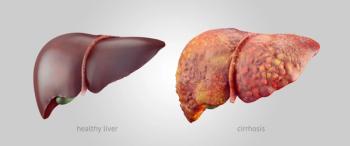
A recent review highlights the gaps in clinical guidelines and treatment approaches for metabolic dysfunction-associated steatohepatitis (MASH) and looks ahead to the future promise of glucagon-like peptide 1 (GLP-1) receptor agonists for liver conditions.