
A review and meta-analysis found mirvetuximab soravtansine to have encouraging efficacy and a tolerable safety profile in patients with recurrent ovarian cancer with folate receptor alpha (FRα) expression.

A review and meta-analysis found mirvetuximab soravtansine to have encouraging efficacy and a tolerable safety profile in patients with recurrent ovarian cancer with folate receptor alpha (FRα) expression.
The overall decline was mainly driven by decreased incidences of epithelial cancers in the ovaries, fallopian tubes, or peritoneum, as well as a decrease in the number of patients diagnosed with epithelial cancer, especially in advanced stages.
In patients with high-grade ovarian cancer, an increased proportion of CD3+ CD137+ tumor infiltrating lymphocytes (TILs) correlated with favorable overall survival outcomes. However, proportions of CD3+ or CD3+ CD8+ TILs did not show significant associations with OS.
A predictive model utilizing serum metabolic profiles was able to distinguish ovarian cancer from control samples with 93% accuracy, according to a new study.

In a cohort with more than half a million women with endometrial, ovarian, or cervical cancer, less than 1% were enrolled in a clinical trial, and more than 85% of enrolled women were White.

The study provides proof of principle for a novel approach to early detection of ovarian cancer based on an assessment of genomic instability patterns in DNA from Papanicolaou (Pap) smears.

The January 2024 guidelines update also changed mirvetuximab soravtansine plus bevacizumab from a category 2B recommendation to a category 2A recommendation for platinum-resistant, FRα-expressing tumors.
High Bone Morphogenetic Protein 7 (BMP7) expression was significantly associated with aggressive phenotypes, including advanced grade, International Federation of Gynecology and Obstetrics stage, residual disease, and adverse overall survival.
Researchers noted that it remains unclear whether cytomegalovirus (CMV) infection is a specific cause of worsened cancer-related cognitive impairment (CRCI), or if it is a biomarker for immune suppression.
The use of olaparib as a monotherapy or in combination with cediranib did not provide a statistically significance overall survival (OS) benefit compared with standard of care platinum-based chemotherapy in patients with recurrent, platinum-sensitive ovarian cancer.
An artificial intelligence (AI)–based prediction model correctly predicted outcomes for 78% of patients with high-grade serous ovarian carcinoma, with an accuracy of 80%.
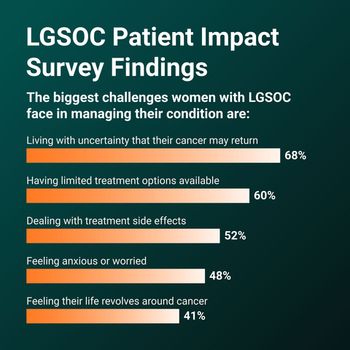
An online survey of patients from multiple countries has shed light on their challenges in getting proper care for ovarian cancer.
After implementing a multidisciplinary surgical approach, researchers found that use of the new approach, residual disease, and age were all independent predictors of overall and progression-free survival for patients with ovarian cancer.

The phase 3 FLAMES trial results demonstrated an improvement in progression-free survival with senaparib monotherapy vs placebo, regardless of patient subgroup, in patients with newly diagnosed, advanced ovarian cancer.
Results from the OPEB-01/APGOT-OV4 trial highlight the potential benefits of using olaparib, pembrolizumab, and bevacizumab as a triplet maintenance therapy for patients who have responded to chemotherapy after experiencing platinum-sensitive recurrence in ovarian cancer.
The European Medicines Agency (EMA) has accepted a marketing authorization application seeking the approval of mirvetuximab soravtansine-gynx for the treatment of patients with folate receptor alpha–positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer.

A study assessing an experimental cell-free DNA myelination liquid biopsy test was found to be 91% accurate at identifying early-stage epithelial ovarian cancer.

Non–guideline-concordant care for ovarian cancer was associated with higher all-cause and cancer-specific mortality, increased health care utilization, and increased Medicare expenditures, highlighting opportunities for improving cancer care in this vulnerable group.
The preoperative risk assessment algorithm can identify ovarian lesions with a strong likelihood of being non-cancerous and suitable for ovary-preserving surgery.

More ovarian cancer drug approvals and treatments have become available since 2014 than in the preceding 60 years combined, emphasized Rebecca Brooks, MD, UC Davis Health.
The FDA has granted fast track designation to IDE161 for the treatment of adult patients with advanced or metastatic ovarian cancer harboring germline or somatic BRCA1/2 mutations who are platinum resistant and have received prior treatment with antiangiogenic and poly-ADP ribose polymerase, or PARP, inhibitor therapies.

Family history is probably the biggest risk factor that patients need to know about, said Rebecca Brooks, MD, UC Davis Health.

Women with ovarian, breast, skin, and uterine cancers were found to have significantly higher levels of per- and polyfluoroalkyl substances (PFAS), phenols, and parabens in their bodies.

Patients with low-grade serous ovarian cancer appear to have worse survival outcomes following treatment with neoadjuvant chemotherapy (NACT).
The treatment combined adoptive T-cell therapy with a personalized cancer vaccine and was found to be safe in a small cohort of 17 patients.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
