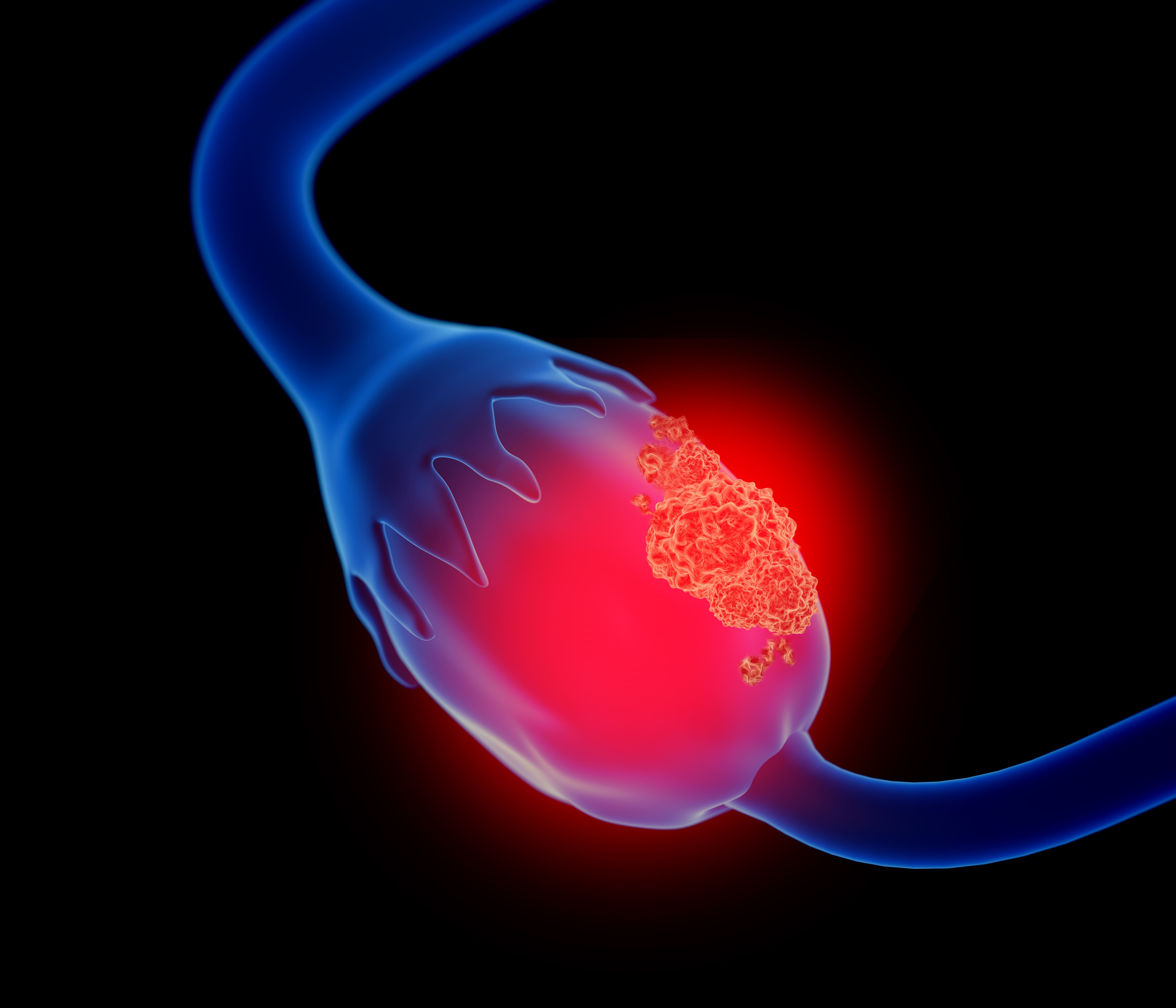
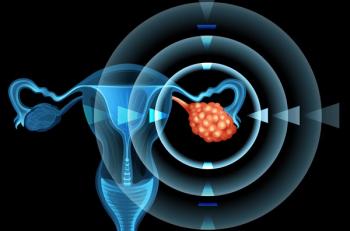
The platinum drugs most effective in treating ovarian cancer are in limited supply due to the drug shortage; the government proposes a plan to prevent hospitals from redistributing Medicaid money; physicians ask for help in treating the high number of children with mental illness coming to the emergency department.