With clear differences at baseline and subsequent declines seen in a linear pattern, the researchers suggested peak expiratory flow may be a suitable outcome measure for longitudinal assessment of respiratory muscle strength in these patients.

With clear differences at baseline and subsequent declines seen in a linear pattern, the researchers suggested peak expiratory flow may be a suitable outcome measure for longitudinal assessment of respiratory muscle strength in these patients.

Improvements, explained the researchers, were mainly driven by younger patients, with smaller improvements seen in older patients.

According to researchers, their findings warrant close surveillance of weight and BMI/age z-scores for patients with type 2 spinal muscular atrophy (SMA).

The researchers investigated the role that economic evaluation and budget impact analysis plays in reimbursement decisions for nusinersen, collecting data from countries across the globe.

Through the use of patient testimonials as well as analysis of data from a study of patients with spinal muscular atrophy (SMA), researchers were able to describe patient-centered “meaningful outcomes” in the 32-item Motor Function Measure.

Coupled with these interventions for treating spinal muscular atrophy (SMA) is an emphasis on diagnosing the disease as early as possible to ensure optimal clinical outcomes for patients.

Researchers outlined recommended treatment plans to build back effective levels of nusinersen following treatment gaps.
Researchers compiled data from studies of oral Evrysdi (risdiplam), Spinraza (nusinersen), and Zolgensma (onasemnogene abeparvovec), finding evidence that risdiplam may be a favorable alternative to nusinersen for type 1 SMA.

According to researchers, this case of a patient with spinal muscular atrophy (SMA) from Indonesia was the first to use a collaborative, multidisciplinary approach that involved obstetric, neurology, pediatric, and anesthesiology departments at a hospital.

Risdiplam is approved for children older than 2 months with spinal muscular atrophy (SMA); an expanded approval would include the youngest infants before they show symptoms.

Among the 3 methods used across 478 samples included in the study, next-generation sequencing provided the most favorable results.

Over the 24-year period, there were 57 spinal muscular atrophy diagnoses in 53 families, and a disease birth prevalence of 1 per 8286 births in Estonia.

Outside of survival motor neuron (SMN) treatment, there are several alternatives used in cases where SMN approaches are not appropriate for treating spinal muscular atrophy (SMA).
Across most questionnaire domains, patients reported improvements to their quality of life (QOL) while receiving treatment for spinal muscular atrophy (SMA) with nusinersen.

Based on the data from over 5000 patients with spinal muscular atrophy (SMA) and 50,000 age-matched controls, patients with SMA had a nearly 1.8-fold higher mortality rate.
The respiratory complications of spinal muscular atrophy are well-researched, but a new study provides insight into nonrespiratory complications that may be just as crucial.

Patients with spinal muscular atrophy often present with severe scoliosis but face an elevated risk of complications from corrective surgery.

The top 5 most-read spinal muscular atrophy stories of 2021 on AJMC.com covered a variety of topics concerning the disease, including cost differences, the potential for combination therapies, and identifying biomarkers for treatment.

If further studies confirm a benefit by using Multispectral Optoacoustic Tomography (MSOT), the technology can help forge a path toward a noninvasive, bedside, nonionizing approach to early visualization of disease as well as evaluation of disease burden and disease progression in patients.
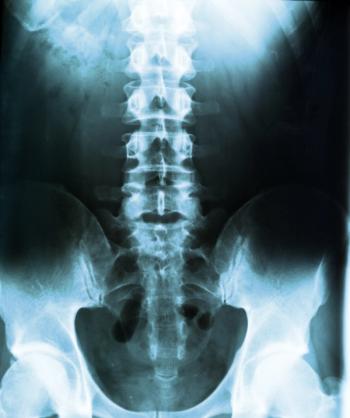
A case series of corrective surgeries in children with severe scoliosis due to spinal muscular atrophy (SMA) type 1 showed a positive success rate, but with significant postoperative complications.

Testing for spinal muscular atrophy in an Australian newborn screening program identified patients across socioeconomic and cultural demographics, mitigating inequity and providing patients with access to multidisciplinary treatment.

Sensory nerve action potential amplitudes decreased as patients aged in a cohort of spinal muscular atrophy (SMA) type 1 patients compared with a healthy, age-matched control group.

Authors of a systematic literature review concluded more research is needed to better understand the financial burden of spinal muscular atrophy (SMA).

A recent review provides insight into the clinical progression of treatment-naive patients with spinal muscular atrophy type 3 who have lost ambulation.

A retrospective observational cohort study of patients ranging in age from infants to adults provides real-world evidence that nusinersen is safe and effective for those with SMA1.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
