
The top 5 most-read stories about spinal muscular atrophy (SMA) of 2020 on AJMC.com focused on a new treatment option for SMA and whether gender or gene mutations have any effects on the disease.

The top 5 most-read stories about spinal muscular atrophy (SMA) of 2020 on AJMC.com focused on a new treatment option for SMA and whether gender or gene mutations have any effects on the disease.
Researchers review pulmonary function testing measures that span 4 categories that can assess the respiratory muscle dysfunction that happens in diseases like spinal muscular atrophy (SMA).
Researchers found abnormal coherence patterns in survivors of childhood polio.

Long-term follow-up of the treatment showed that while it has a favorable safety profile, the treatment does not offer significant efficacy for patients with spinal muscular atrophy (SMA) type 2 and non-ambulant type 3.

Between the first introduction of population carrier screening in 2008 and its expansion to the full Israeli population in 2013, the mean rate of prenatal diagnoses per year was 4.66, and this rose to 7.75 between 2014 and 2017, a 66% increase.

For infants, the main driver of these increased costs came from inpatient visits vs outpatient visits among children and juveniles.

Treatment with risdiplam may not elicit ophthalmologic toxicity in older patients, as well as infants with immature retinas.
Researchers have published their postmortem report on a case of spinal muscular atrophy type 3 complicated by superficial siderosis, offering details on their neuropathological and molecular findings.
The compiled results revealed that hepatotoxicity related to the gene therapy typically presented as cholestatic, which could be prophylactically treated with prednisolone.
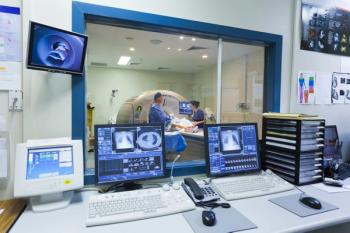
Using DIXON, T2 mapping, and diffusion tensor imaging, the researchers obtained quantitative MRI for 31 patients with spinal muscular atrophy (SMA) types 2 and 3.
Following 6 injections of nusinersen, respiratory muscle performance was significantly better among the treated patients compared with age‐matched historical controls.

At the time of treatment, there was just 1 paper documenting the use of nusinersen in a case of more mild type 0 spinal muscular atrophy(SMA).

Researchers have identified additional mutations in both SMN1 and other genes, some of which may be associated with the onset of spinal muscular atrophy (SMA).
The study emphasizes the importance of school support and other psychosocial interventions considering specific deficits across adaptive skills in patients with neuromuscular diseases (NMDs).
The researchers of the review discuss the reported impact of novel therapies for spinal muscular atrophy (SMA) and highlight the need for long-term data and monitoring of respiratory outcomes.

Jill Jarecki, PhD, chief scientific officer at Cure SMA explained the current data and potential for gender to impact the severity spinal muscular atrophy (SMA).
The study offers the first de novo cost-effectiveness model of these patients in the United States, according to the researchers.

Early treatment of infants with spinal muscular atrophy (SMA) can drastically improve motor function for patients, explained Jill Jarecki, PhD, chief scientific officer at Cure SMA.

A new study is underscoring the importance of diagnostic testing for patients with spinal muscular atrophy (SMA) to ensure early diagnosis of the disease.

Current treatment options spinal muscular atrophy (SMA) are available for all types of the disease regardless of motor strength, explained Jill Jarecki, PhD, chief scientific officer at Cure SMA, and Mary Schroth, MD, FAAP, FCCP, chief medical officer at Cure SMA.
Analyzing data from a phase 2 study of patients with type 2 or non-ambulant type 3 spinal muscular atrophy (SMA,) researchers found that the assessment is a valid, reliable, and responsive assessment of motor function ability for these patients.

Combination treatments may be the future for treating patients with spinal muscular atrophy (SMA) at all stages and ages, explained Jill Jarecki, PhD, chief scientific officer at Cure SMA and research director of TREAT-NMD Neuromuscular Network.

A recent study about the impact of spinal muscular atrophy (SMA) on patients and caregivers may help to illustrate the impact of future improvements as new therapies take hold, but more targeted SMA quality-of life-outcome measures may be needed to fully the changes.

Increasing at-home access to care and treatment options for spinal muscular atrophy (SMA) proved to be helpful during the coronavirus disease 2019 (COVID-19) pandemic, explained Mary Schroth, MD, FAAP, FCCP, chief medical officer at Cure SMA.

At the time of the 2-year analysis, 88% of the 17 infants who received the therapeutic dose of risdiplam for spinal muscular atrophy (SMA) were alive and did not require permanent ventilation.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
