
Tara Carlisle, MD, PhD, University of Colorado, discusses how coordinated care efforts and frequent safety monitoring are transforming early Alzheimer disease management.

Tara Carlisle, MD, PhD, University of Colorado, discusses how coordinated care efforts and frequent safety monitoring are transforming early Alzheimer disease management.

Ken Cohen, MD, FACP, discusses the importance of expanding cognitive screening for seniors.

Experts discuss the importance of initiating blinatumomab treatment for acute lymphoid leukemia in academic centers for optimal patient care.
Kelley L. Julian, PharmD, BCOP, discusses optimizing myeloma care, addressing barriers in access to intravenous immunoglobulin and innovative therapies at Huntsman Cancer Institute.

Clinician's role in breast cancer care and access with reproductive and urinary oncologist Lakshminarayanan Nandagopal, MD

Advancements in breast cancer care and treatments with reproductive and urinary oncologist Lakshminarayanan Nandagopal, MD.

Oranus Mohammadi, MD, outlines how antibody-drug conjugates are transforming breast cancer treatment across subtypes and discusses her approach to sequencing high-cost targeted therapies within payer and clinical practice constraints.

Matias Sanchez, MD, a hematologist-oncologist at University of Illinois Health, discussed the latest advancements in value-based multiple myeloma care, including minimal residual disease strategies and innovative targeted therapies.

Oranus Mohammadi, MD, discusses the emerging applications of circulating tumor DNA (ctDNA) in breast cancer care and emphasizes the importance of clear communication to help patients navigate uncertain or anxiety-provoking biomarker test results.

Joanne Mortimer, MD, FACP, FASCO, discusses the practical applications and limitations of circulating tumor DNA (ctDNA) testing in breast cancer, highlighting its role in guiding targeted therapy, challenges in patient communication and payer coverage, and unique barriers for male patients.

Jorge Nieva, MD, explores the challenges of translating biomarker testing into treatment decisions for non–small cell lung cancer (NSCLC), the role of repeat testing in detecting resistance mutations, and the importance of equitable access to molecular diagnostics in value-based care settings.

Jorge Nieva, MD, highlights the critical role of molecular testing in non–small cell lung cancer (NSCLC) care, while addressing barriers such as limited tissue samples, delayed turnaround times, and the need for faster, more accessible diagnostic technologies.

Yale Podnos, MD, MPH, FACS, discusses strategies to address social determinants of health in oncology, improve clinical trial enrollment for underserved communities, and leverage value-based care models to reduce financial toxicity and ensure equitable cancer care.

Daniel Virnich, MD, highlights the need for proactive social determinants of health screening, language-inclusive clinical trial practices, value-based treatment decisions, and policy reforms to improve equitable access to cancer care.

Lauren Antrim, MD, of City of Hope Cancer Center Duarte, emphasized the need for more evidence to guide optimal immunotherapy duration and sequencing in in non–small cell lung cancer (NSCLC), highlighting ongoing trials and the potential role of ctDNA in tailoring treatment strategies.

Lauren Antrim, MD, emphasized the need to balance safety, efficacy, and financial considerations when managing immune checkpoint inhibitors in NSCLC, underscoring the importance of patient-centered discussions and ongoing trials to refine treatment duration strategies.

The definition of comprehensive non-small cell lung cancer (NSCLC) testing is rapidly evolving with new biomarkers and therapies, according to Julia Rotow, MD, creating added challenges for community practices.

Cutting-edge therapies can help move the treatment landscape forward, but basic treatment and prevention, such as smoking cessation, are still valuable means of addressing non–small cell lung cancer (NSCLC).

Jonathan Thompson, MD, MS, explains how financial, insurance, and socioeconomic barriers limit equitable access to biomarker testing and advanced therapies, underscoring the need for provider advocacy and systemic support.

Treatment challenges that Anasuya Gunturi, MD, PhD, encounters in her work at Lowell General Hospital include language differences and confusion about scheduled appointments.
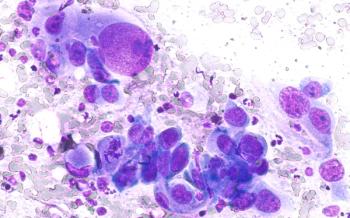
In advanced non–small cell lung cancer (NSCLC), discontinuing immunotherapy after 2 years can maintain durable responses while reducing financial and toxicity burdens, with decisions guided by residual disease testing and shared decision-making, explained Jonathan Thompson, MD, MS.

Making treatment for non–small cell lung cancer (NSCLC) accessible to a wide range of the population can help to improve outcomes from and knowledge of the condition.
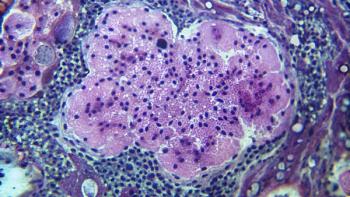
Jonathan Thompson, MD, MS, explains that adjuvant immunotherapy benefits patients with early-stage lung cancer with incomplete neoadjuvant response, while treatment decisions in the adjuvant setting must weigh efficacy, toxicity, and limited evidence.
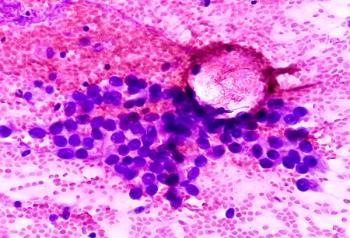
Jonathan Thompson, MD, MS, highlighted that reducing delays in molecular testing and treatment initiation is critical for improving lung cancer outcomes, and that clinical trial data suggest immunotherapy duration can often be safely de-escalated in patients who achieve a complete pathologic response.

A panel held during the Institute for Value-Based Medicine event in Chicago on August 14, 2025, included discussions of access to biomarker testing and perioperative treatment in patients with non–small cell lung cancer (NSCLC).
Jonathan Thompson, MD, MS, emphasized that broader molecular testing in early-stage non�–small cell lung cancer (NSCLC) is essential to guide perioperative treatment decisions, while selective retesting at progression can identify resistance mutations or new targets to optimize value-based care.

Emilie Aschenbrenner, PharmD, BCOP, outlines how Froedtert Health and the Medical College of Wisconsin use outpatient-based care models, standardized protocols, and collaborative partnerships to improve cost-effectiveness, accessibility, and patient experience.

Clayton Irvine, PharmD, MBA, MS, discusses how pharmacists are essential to advancing payer–provider collaboration in value-based care by shaping formularies, standardizing drug reviews, leveraging real-world evidence, and leading pilot programs to optimize patient outcomes and cost-effectiveness.

Despite its potential, incorporating new treatments like T-DXd into a first-line setting faces several barriers, explains Michael Hassett, MD, MPH, chief quality officer at Dana-Farber Cancer Institute in Boston.

Emilie Aschenbrenner, PharmD, BCOP, highlights how measurable residual disease (MRD) testing can support value-based care in multiple myeloma by guiding personalized treatment intensity, potentially reducing costs and improving outcomes.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
