
The 64th American Society of Hematology (ASH) Meeting & Exposition will run Saturday through Tuesday in New Orleans, Louisiana, at the Ernest N. Morial Convention Center.

The 64th American Society of Hematology (ASH) Meeting & Exposition will run Saturday through Tuesday in New Orleans, Louisiana, at the Ernest N. Morial Convention Center.

There is new data coming out on complementary and alternative therapies for dementia, including supplements, mindfulness techniques, exercise, music therapy, and more, said Kalin Clifford, PharmD, BCGP, BCPS, FASCP, associate professor, Geriatrics Division, Texas Tech University Health Sciences Center, Jerry H. Hodge School of Pharmacy.

Once patients with HIV start treatment, pharmacists can play a key role in addressing patient accessibility and affordability of HIV treatments and promoting adherence, said Dena Behm Dillon, PharmD, AAHIVP, HIV clinical pharmacy specialist, University of Iowa Health Care.

There are many risk factors for developing postoperative nausea and vomiting (PONV), but a simplified risk score can help with assessing those risk factors, said Jawad N. Saleh, PharmD, BSPharm, BCCCP, BCPS, clinical manager of pharmacy services, Hospital for Special Surgery.

A review of available literature, although limited, has pointed to a link between pediatric psoriasis and anxiety and depression. Previous research has made a clear association between psoriasis and mood disorders in adults.

Pharmacists can play an important role in dispelling myths about vaccines, as well as identifying patients who are eligible for vaccinations that they haven’t yet received, said Jacinda C. Abdul-Mutakabbir, PharmD, MPH, AAHIVP, assistant professor of pharmacy practice, Loma Linda University.

The CDC reported that the number of people who were hospitalized with the flu nearly doubled during the week of Thanksgiving; contraception for people producing sperm is finding promising results in clinical trials; marijuana use in children has risen 245% in the last 20 years.
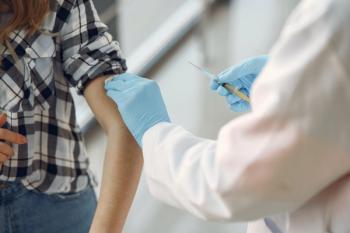
The monthly volume of human papillomavirus (HPV) vaccine doses administered have returned to the level observed prior to the COVID-19 pandemic among children in an integrated health care system in California, but HPV vaccine coverage remains lower compared with prepandemic levels.

Liz Lightstone, MBBS, PhD, FRCP, professor of renal medicine for the Faculty of Medicine, Imperial College London, discussed the influence of genetics and background on the care management of lupus nephritis.

Coverage from the Institute for Value-Based Medicine® session with Astera Cancer Care in Edison, New Jersey, held November 3, 2022.

Female allopathic medical students pursuing careers in dermatology were less likely than those pursuing other specialties to be from racial and ethnic groups underrepresented in medicine or be a sexual minority, with a lack of interest in underserved care and public health shown overall.

Marie A. Chisholm-Burns, PharmD, PhD, MPH, MBA, FCCP, FASHP, FAST, executive vice president and provost at Oregon Health and Science University, is receiving the award, which is presented to individuals who demonstrate excellence in pharmacy practice leadership.

Melissa O'Connor, PhD, MBA, RN, FGSA, FAAN, endowed professor in Community and Home Health Nursing, M. Louise Fitzpatrick School of Nursing, Villanova University, and director, Gerontology Interest Group, noted that a comprehensive assessment of each patient is necessary to develop individualized care plans that can achieve better outcomes and keep older adults in the home setting.
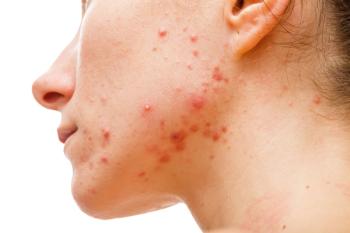
Patient-reported outcome (PRO) measures were included in approximately one-half of acne vulgaris and rosacea randomized controlled trials, despite their utility in capturing the patient perspective.
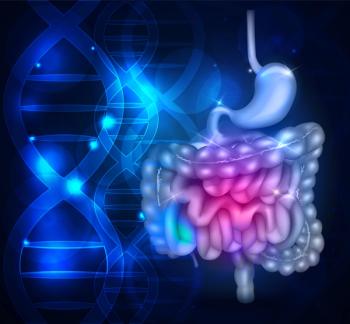
People with a gastrointestinal biopsy of normal mucosa or nonspecific inflammation were shown to be at increased risk of Alzheimer disease and Parkinson disease.

Early life exposure to antibiotics, particularly during weeks 2, 3, and 4 of life, was associated with a decreased risk of atopic dermatitis development.

Only 2% of patients with primary sclerosing cholangitis (PSC) received a cholangiocarcinoma diagnosis, and the diagnosis was not early enough for there to be a survival benefit.
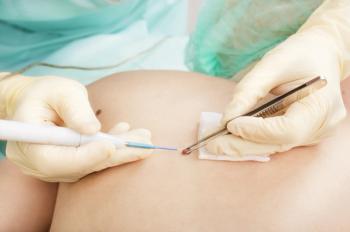
Malignant cutaneous melanoma outcomes were investigated as they relate to diagnosis delay and potential influence from socioeconomic and demographic factors in Brazil, where skin cancer diagnoses represent 30% of all cancer diagnoses.

Investigators also confirmed that FGFR2 fusion–positive tumors have a higher expression of FGFR2 but were not associated with higher expression of FGFR1, FGFR3, or FGFR4.

Researchers pulled data from the FDA’s Adverse Event Reporting System to analyze cardiac arrhythmia–related outcomes among patients on a mono or combination regimen for cancer treatment that included immune checkpoint inhibitors (ICIs).

Researchers underscore a need for prospective data, noting that women with chronic kidney disease (CKD) tended to have higher rates of preterm delivery and lower birthweight.

Researchers found that different factors can negatively impact the Dermatology Life Quality Index scores for adults and adolescents with acne.
Melissa O'Connor, PhD, MBA, RN, FGSA, FAAN, endowed professor in community and home health nursing, M. Louise Fitzpatrick School of Nursing, Villanova University, and director, Gerontology Interest Group, addressed barriers related to access, cost, and knowledge impeding technology use in home health.

Data on empagliflozin in chronic kidney disease (CKD) showed the drug had similar efficacy across subgroups, but more data is needed to really understand the benefit of the drug in CKD, said Jennifer Green, MD, professor of medicine at Duke University School of Medicine, member of Duke Clinical Research Institute, and EMPA-KIDNEY collaborator.

Abstracts show that institutions can make internal changes to drive the use of biosimilars and that successful biosimilar-to-biosimilar switching is based on patient-related factors.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
