Clinical
Latest News
Latest Videos

CME Content
More News
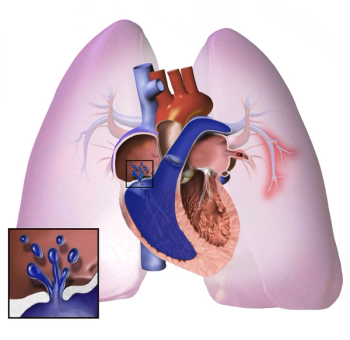
Compared with treprostinil, selexipag carried a 47% reduced risk of pulmonary arterial hypertension (PAH)-related hospitalization and 46% reduced risk of all-cause hospitalization.

Research behind the therapy could lead to other discoveries about heart disease as well as the aging process.

Most patients who are diagnosed with acute myeloid leukemia are in middle or old age. The relatively small number who are diagnosed earlier in life can be subject to long-term health risks due to their treatment.

Authors in the Journal of Clinical Oncology discuss hypotheses on the differences in mutations and implications for treatment with PARP inhibitors.
Using DIXON, T2 mapping, and diffusion tensor imaging, the researchers obtained quantitative MRI for 31 patients with spinal muscular atrophy (SMA) types 2 and 3.
Mounting evidence is supporting the use of poly ADP-ribose polymerase (PARP) inhibitors in prostate cancer, according to a new study.

The study compared females and males who suffer from migraines and other painful conditions who took part in a national health survey in Spain.

Results of the artificial intelligence (AI) model were consistent no matter which immune checkpoint inhibitor (ICI) therapy patients received, suggesting that some biomarkers are not necessarily specific to the checkpoint target.

The mortality risk differs for the 3 endothelin receptor antagonists approved to treat pulmonary arterial hypertension (PAH) in elderly patients; however, a direct comparison in a controlled trial is still needed to confirm results.
The oral selective inhibitor of nuclear export could provide a new therapeutic avenue in daratumumab-naive patients with relapsing refractory multiple myeloma (RRMM).
A new analysis of CREDENCE data showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor worked in advanced disease and without causing acute kidney injury.

Clarence Moore, PharmD, BCPS, BCOP, assistant professor at Shenandoah University in Ashburn, Virginia, discussed the potential complications for patients with beta thalassemia associated with iron overload and the significance of chelation therapy.
Drawing on data from 6 randomized controlled trials accounting for over 2200 patients, the researchers the researchers compared the efficacy and safety of olaparib, rucaparib, and niraparib.

Women filling a prescription for female hormone therapy (FHT), and presumably taking FHT, are not at increased risk of retinal artery or retinal vein occlusions.
Results from a phase 3 study of Xywav in adult patients with idiopathic hypersomnia exhibited its potential to address this unmet medical need.
While migraine was more manifest in some patients during the pandemic, others were able to adopt healthier habits, said Peter McAllister, MD, a neurologist, board certified headache specialist, and medical director of the New England Institute for Neurology and Headache.

The genetic determinants of sleep and migraine partly overlap, suggesting sleep disturbances may causally influence migraine etiology and could be targets for migraine treatment, according to results of a Mendelian randomization study.

An abnormal red reflex finding most likely reflects an underlying ocular pathology in infants, but finding a normal red reflex during screening does not altogether exclude ocular disease.

US Rheumatologists Reluctant to Switch to Biosimilars for Patients Doing Well on a Reference Product
Although half of US rheumatologists have patients for which nonmedical switching has been suggested, only 35% were likely to switch patients with rheumatoid arthritis doing well on a reference product to a biosimilar.
For patients with polycythemia vera (PV), there are no differences in recommendations for preventing complications when using ruxolitinib than for myelofibrosis (MF).
A study looking at chronic obstructive pulmonary disease (COPD) and coronary heart disease found that inhaled corticosteroids (ICS) reduced future cardiac events in patients not known to have coronary heart disease.

At the time of treatment, there was just 1 paper documenting the use of nusinersen in a case of more mild type 0 spinal muscular atrophy(SMA).

Chimeric antigen receptor (CAR) T cells can be highly effective, but the durability of the therapy has been lacking in many patients with hematological malignancies. Many efforts are underway to fix this problem.

The FIDELIO-DKD program is perhaps just the beginning of seeing how finerenone can be used, said Rajiv Agarwal, MD, MS, FASN, a professor of medicine at Indiana University School of Medicine and a staff physician at the Veterans Affairs Medical Center in Indianapolis, Indiana.

Researchers have identified additional mutations in both SMN1 and other genes, some of which may be associated with the onset of spinal muscular atrophy (SMA).