
Clinical
Latest News
Latest Videos

CME Content
More News





Patients are significantly overestimating the pain they will feel after surgery, indicating that providers can do a better job of educating patients on what they can expect.

A non-fasting test to measure low-density lipoprotein (LDL) cholesterol has many advantages for patients and physicians, but payers can also see some benefits, explained Eliot A. Brinton, MD, FAHA, FNLA, president of the Utah Lipid Center.

Coverage of our peer-reviewed research and news reporting in the healthcare and mainstream press.

Chemotherapy and radiation therapy should be the first line of treatment for certain prostate cancers and lymphomas with a major genetic weakness, according to researchers at the University of Virginia School of Medicine.

VA Secretary David Shulkin wants the private sector to play a larger role in veterans' healthcare; Arizona's Supreme Court ruled in favor of a component to keep the state's Medicaid expansion; Acorda ends drug development of tozadenant for Parkinson's disease.
Most US adults would prefer to find other ways to manage their pain, such as neck or back pain, before taking prescribed pain medication, a Gallup research brief found.

A recent abstract presented at the American Society of Clinical Oncology (ASCO) annual meeting compared 2 risk models for patients with intermediate chemotherapy-induced neutropenia (CIN) risk and compared them to guidelines from ASCO and the National Comprehensive Cancer Network to determine when colony-stimulating factor should be ideally used to prevent CIN. ​​​​​​
With an expansion that includes immunotherapy combination treatments, the American Society of Clinical Oncology (ASCO)’s Targeted Agent and Profiling Utilization Registry (TAPUR) Study has now grown to 500 participants and 16 therapies.

The new hypertension guidelines made major changes to the classification of blood pressure, in general, and changed the name of one category to convey more importance, explained Robert Carey, MD, MACP, professor of medicine and dean emeritus at the University of Virginia.

Recent results from a team-based, scalable intervention to promote medication adherence highlighted that the relationship between adherence and clinical outcomes is not always clear cut, said Niteesh Choudhry, MD, PhD, associate professor at Harvard Medical School.

The outcomes in the COMPASS trial of rivaroxaban to treat patients with peripheral artery disease have been very positive, and a new analysis has looked at the cost impact of bringing the drug to market, explained Andre Lamy, MD, MHSc, FRSC, a cardiac surgeon with the Population Health Research Institute in Hamilton, Ontario, Canada.
Chronic obstructive pulmonary disease (COPD) is characterized by persistent and progressive airflow limitation, and a new study examined the factors responsible for a high St George’s Respiratory Questionnaire for COPD based on severity of airflow limitation.

The time a patient is in a doctor's office represents a very small window of the body normally, which makes out-of-office blood pressure measurements important to confirm diagnosis of hypertension, explained Paul Whelton, MD, MSc, professor of global public health at Tulane University.
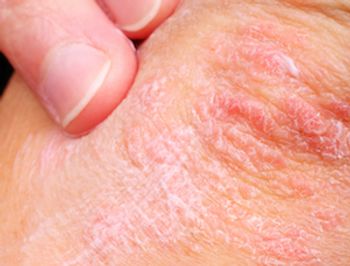
Patients with psoriasis and with rheumatoid arthritis (RA) are often treated with similar drugs, but those with psoriasis are at a higher risk for serious liver disease, according to a new study in the Journal of Investigative Dermatology.

As oncology moves toward more deep diagnostic testing and as standard of care continues to quickly evolve, technology advancements are necessary to continue to improve patient access to clinical trials, explained Amy Abernethy, MD, PhD, the chief medical officer, chief scientific officer, and senior vice president of oncology at Flatiron Health.

The EMPA-REG trial has been a big step forward for clinicians being able to put patients with type 2 diabetes onto treatment that also reduces cardiovascular disease risk, which is the primary cause of death in these patients, explained Eliot A. Brinton, MD, FAHA, FNLA, president of the Utah Lipid Center.

Roger S. McIntyre, MD, FRCPC, professor, Departments of Psychiatry and Pharmacology, University of Toronto, and of the Head, Mood Disorders, Psychopharmacology Unit, University Health Network, discussed the contributing factors and effects of misdiagnosing or inappropriately treating patients with antidepressants.

A session at the 2017 NEI Congress emphasized the importance of making an accurate diagnosis in patients presenting symptoms of depression.

Only 1 in 3 patients will achieve remission on their first antidepressant, and 67% of patients require 4 antidepressant trials before symptoms remit, said Thomas L. Schwartz, MD, during a session on strategies for switching, combining, or augmenting treatments for patients with major depressive disorder.
A specific mutation has been linked with acquired resistance in immunotherapy drugs used to treat non–small lung cancer (NSCLC).



















