Dr Lawrence Eichenfield Discusses Positive Results of Roflumilast Cream 0.05% in Pediatric Patients With AD
Lawrence Eichenfield, MD, of Rady Children's Hospital - San Diego, discusses the implications of the findings from INTEGUMENT-PED, the phase 3 study assessing the efficacy and safety of once-daily roflumilast cream 0.05% in pediatric patients with atopic dermatitis (AD).
This content was produced independently by The American Journal of Managed Care® (AJMC®) and is not endorsed by the American Academy of Dermatology.
At the
In this interview with AJMC, Eichenfield says that roflumilast cream 0.05% had "very good results" in pediatric patients with AD in terms of efficacy and safety.
Transcript
Can you provide an overview of the primary findings from INTEGUMENT-PED, the phase 3 study assessing the efficacy and safety of once-daily roflumilast cream 0.05% in pediatric patients with AD?
First of all, roflumilast is a topical PDE-4 [phosphodiesterase-4] inhibitor, and this drug is approved right now at a 0.3% strength for psoriasis as a cream and as a foam for seborrheic dermatitis.
This study fits into the earlier portfolio of studies looking at roflumilast cream for atopic dermatitis. There had been a study in patients 6 years of age and older through adults, studying roflumilast 0.15% cream application for atopic dermatitis.
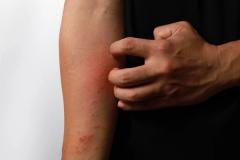
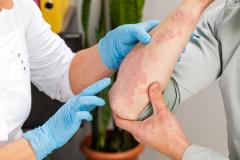
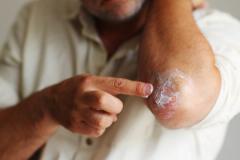
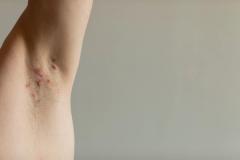
This is the study of patients 2 to 5 years of age with mild to moderate atopic dermatitis, using a formulation of roflumilast cream, which is 0.05%. The study was done in a typical fashion and showed very good results in terms of the core standard end points that we look for, for both efficacy and safety, as a topical for atopic dermatitis in this pediatric population.
What were the key inclusion criteria for selecting participants in this study? How representative were they of the pediatric population with AD?
Patients in this study had to be 2 to 5 years of age with mild to moderate atopic dermatitis. They had to have a minimum body surface area of 3%, and they had to have an EASI [Eczema Area and Severity Index] score that was 5 or greater. That's a pretty typical population within this age group, for 2 to 5 year olds. We have a lot of mild or moderate atopic dermatitis in that subgroup—the 3% body surface area—it's just not including those with little focal area of atopic dermatitis. Baseline EASI was consistent with that.
So, it doesn't represent all patients, just within that little age group, but it's a pretty reasonable inclusion criteria for much of the eczema that we see in younger children.
Could you provide insights into the safety profile of roflumilast cream observed during the trial? Were there any unexpected adverse events or safety concerns in this pediatric population?
This study was using the drug as a daily application as compared to vehicle cream for a 4-week time period. There was a very low dropout rate during the study, something that we like to see in topical studies so that we don't have a sense that patients abandoned the study because of problems with either safety or tolerance, which are, I think, the 2 aspects when it comes to a topical that we're looking at.
The drug appeared to be quite successful from a safety standpoint. The treatment adverse events, there was 1 serious adverse event. It was cellulitis within a few days of the study, not considered to be associated with a drug; no other serious adverse events in either group. The rate of immersion adverse events that led to discontinuation was very low, around the 1% range with roflumilast and about twice that, actually, in the vehicle group. There was a little bit of a upper respiratory infection that was seen with roflumilast, and then a little bit of diarrhea and vomiting that was a little higher than the vehicle group; this was generally quite mild and considered unrelated in most of the patients.
When it comes to the tolerability, there were particular tests that were looking at that because that's important in a topical agent. There was a investigated rater local tolerability score that was essentially a 0, 1, 2, 3 score, looking at aspects of redness in the population. It was essentially zero throughout the course of the study, pretty much overlapping curves between the roflumilast and the vehicle cream.
The patient-rated tolerability was really quite good, actually, with the roflumilast cream being considered more tolerable than the vehicle cream, which is interesting, I guess because of the beneficial effects on eczema. That was throughout the course of the study.
Newsletter
Stay ahead of policy, cost, and value—subscribe to AJMC for expert insights at the intersection of clinical care and health economics.