Dr Jonathan Silverberg Discusses Findings on the Safety, Efficacy of Nemolizumab at 48 Weeks
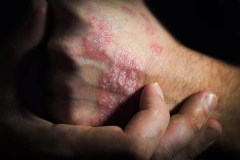
In patients with moderate to severe atopic dermatitis, nemolizumab demonstrated good durability of response, explained Jonathan Silverberg, MD, PhD, MPH, FAAD, of George Washington University School of Medicine and Health Sciences.
This content was produced independently by The American Journal of Managed Care® and is not endorsed by the American Academy of Dermatology.
The results of this study demonstrated good durability of response and even some ongoing improvement with nemolizumab beyond week 16 up to week 48 in patients with moderate to severe atopic dermatitis (AD), says Jonathan Silverberg, MD, PhD, MPH, FAAD, professor of dermatology, director of clinical research and patch testing, George Washington University School of Medicine and Health Sciences.
Transcript
Can you provide an overview of the study and the primary efficacy end points achieved at week 48 in both ARCADIA-1 and ARCADIA-2 studies for patients with moderate to severe AD receiving nemolizumab?
The late-breaking presentation was for data from ARCADIA-1 and ARCADIA-2. These are 2 global, phase 3 randomized control trials that included both adolescents and adults with moderate to severe atopic dermatitis. And the primary efficacy end points and readouts were presented a few months ago at the European Academy of Dermatology and Venereology, which is that initial 16-week treatment period where patients were given either nemolizumab or the placebo.
This late-breaking presentation examined the 32-week maintenance data from these 2 studies. Patients who are responders by either IGA [Investigator Global Assessment] score or EASI [Eczema Are and Severity Index] 75 response to nemolizumab at week 16, we then re-randomized to get ongoing nemolizumab every 4 weeks, a maintenance dose of every 8 weeks, or placebo at every 4-week intervals for another 32 weeks. And all this is in the background of using topical prescription, topical therapy, corticosteroids, or calcineurin inhibitors.
And what we found was that at week 48, so 32 weeks after that 16-week initial read out, that a very high proportion of patients who are responders maintained that response, really on all doses, whether it was the ongoing every 4-week dose or the every 8-week dose, and even for many who were getting the placebo. And that was true whether it was for IGA responses getting to clear/almost clear, EASI 75 responses, 4-point reduction in itch, as well as even sleep responses and quality of life and things like that. So, we're really seeing good durability of response. Even for some of the end points [there’s] a little bit of an ongoing gain of response beyond week 16 out to that week 48 period.
Newsletter
Stay ahead of policy, cost, and value—subscribe to AJMC for expert insights at the intersection of clinical care and health economics.