Dr Jeff Stark: Pain Reduction With Bimekizumab-bkzx in Patients With HS
Jeff Stark, MD, vice president and head of medical immunology, UCB, shares phase 3 study results of bimekizumab-bkzx given for up to 48 weeks in patients with moderate to severe hidradenitis suppurativa (HS).
This content was produced independently by The American Journal of Managed Care® and is not endorsed by the American Academy of Dermatology.
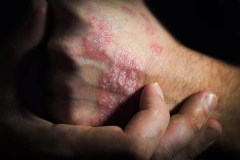
Acute pain is a significant factor for patients with hidradenitis suppurativa (HS) that can be reduced by treating the inflammation associated with this disease, says Jeff Stark, MD, vice president, head of medical immunology, UCB.
Transcript
What specific improvements in skin pain were observed in patients with hidradenitis suppurative (HS) treated with bimuzkizumab-bkzx compared with placebo?
We're very excited about the skin pain results that we're presenting here for bimekizumab at the AAD meeting [American Academy of Dermatology Annual Meeting 2024]. Bimekizumab, as you may know or your listeners may know, is a novel molecule that targets 2 different components of the IL [interleukin]-17 pathway, both IL-17A and IL-17F, both of which we know are potent drivers of inflammation in inflammatory skin diseases like Hidradenitis suppurativa.
The reason I think this is exciting is really 2 reasons. One is that these are longer-term data that helps us to know what to expect as patients are treated with bimekizumab over longer periods of time. Also, because pain is among the most prominent symptoms that patients with HS experience, and so, to be able to speak to their experience with the disease and demonstrate effectiveness of a therapy to reduce that prominent symptom for them is particularly exciting as well.
Newsletter
Stay ahead of policy, cost, and value—subscribe to AJMC for expert insights at the intersection of clinical care and health economics.