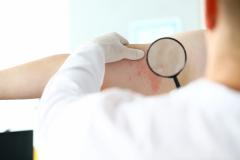
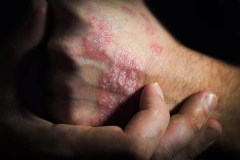
Patients With AD Have More Options With Biologics, JAK Inhibitors, Says Dr Emma Guttman-Yassky
With both biologics and Janus kinase (JAK) inhibitors available to treat atopic dermatitis, providers have more options for patients, said Emma Guttman-Yassky, MD, PhD, FAAD, of Mount Sinai.
This content was produced independently by The American Journal of Managed Care® and is not endorsed by the American Academy of Dermatology.
With both biologics and Janus kinase (JAK) inhibitors available to treat atopic dermatitis, providers have more options for patients, said Emma Guttman-Yassky, MD, PhD, FAAD, the Waldman Professor and System Chair of Dermatology and Immunology at the Icahn School of Medicine at Mount Sinai, the director of the Center for Excellence in Eczema and the Occupational Dermatitis Clinic, and director of the Laboratory for Inflammatory Skin Diseases.
Guttman-Yassky presented on new developments in atopic dermatitis at the annual meeting of the American Academy of Dermatology.
Transcripts
There are a handful of biologics and Janus kinase inhibitors approved now for atopic dermatitis. What are the pros and cons with each of these agents?
First of all, we like to have options for our patients, because always there will be patients that will not mind an injectable or even prefer to take something once every 2 weeks or every 4 weeks. But then there are patients that will say, “Doctor, no matter what I only want an oral.” I think we want options for our patients.
Generally speaking, of course, biologics are more specific, because they target one immune molecule, and that's why in terms of a safety, usually, it's a little bit better because it's less broad. JAK inhibitors, they're still safe, but they are more broad. The more broad you are, of course, you will hit some things—that's the general rule. With that being said, the JAK inhibitors also showed good safety over time. Some of them have 3 and 4 years of safety, and also some of them have been tried in arthritis. So, we have that bulk of safety [data] as well. Some of them are new to dermatology.
Overall, we see a good in good safety. In particular, the diseases we are dealing with—atopic dermatitis and alopecia areata—these are younger patient populations. That's another thing to talk about.
It's exciting times, I think, in general in the field of atopic dermatitis, and also other diseases like alopecia areata, vitiligo, and so on, because we finally have new treatments. In atopic dermatitis in particularly, we are super excited: we have biologics, we have small molecules, we have topicals for the disease. So super exciting times. And finally, doctors and patients have options.
Have you found payer coverage to be more favorable to one or the other?
Of course, with dupilumab, because payers have experience, it's easier to approve. It's already been a while. The newer the agent, you have some struggles in the beginning, but we managed to overcome it. Sometimes you need to apply a few times, there are some prior authorizations, and our staff gets used to it. I guess, for an academic institution, it's a little bit easier. We have people doing these.
Newsletter
Stay ahead of policy, cost, and value—subscribe to AJMC for expert insights at the intersection of clinical care and health economics.