Dr Shawn Kwatra Shares Efficacy, Safety Data of Nemolizumab in Patients With PN
Shawn Kwatra, MD, dermatologist, John Hopkins University, discusses late breaking study results on the long-term efficacy and safety of nemolizumab in patients with prurigo nodularis (PN).
This content was produced independently by The American Journal of Managed Care® and is not endorsed by the American Academy of Dermatology.
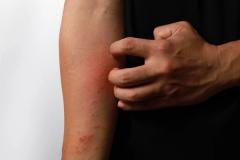
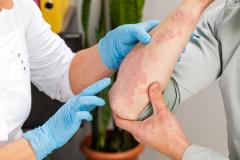
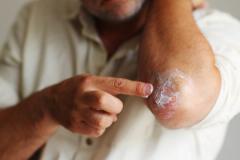
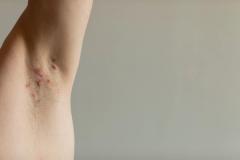
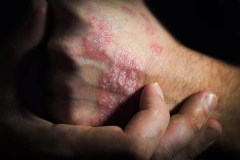
Purirgo nodularis (PN) is a chronic skin disorder that is characterized as multiple, firm, flesh to pink colored papules, plaques, and nodules commonly located on the extensor surfaces of the extremities. The lesions are very pruritic and can occur in any age group,
The study found patients treated with nemolizumab experienced significant improvements in itch, skin lesions, sleep disturbance, and quality of life up to 52 weeks, says Shawn Kwatra, MD, dermatologist, John Hopkins University.
Transcript
Can you provide an overview of the key findings from the interim analysis of the OLYMPIA study regarding the long-term efficacy of nemolizumab in patients with PN?
[In] the nemolizumab long-term extension study, the interim cut that we're seeing today was 52 weeks. And in that 52-week study, we wanted to assess safety and efficacy. Regarding efficacy, 90% of patients achieved a 4-point improvement in their itch. Actually, two thirds of patients were in a relative itch-free state by week 52. There was also very significant improvement in skin lesions. So, 65% to 70% of patients reached an IGA [Investigator Global Assessment] 0 or 1, which denotes clear or almost clear skin by week 52. So significant activity itch, incredible skin findings. And then also, the safety data was consistent with that and earlier phase 3 studies.
What specific measures of efficacy were assessed in the study, and how do the results compare to those observed in previous clinical trials of nemolizumab for patients with PN?
The endpoints that were assess were related to itch, skin lesions, and effects on quality of life and sleep disturbance. And for itch, 90% of patients had a 4-point improvement in their itch, two thirds of patients reaching itch-free state, but it was fast. So, in the group that had never received nemolizumab before, took about 4 weeks for these patients to already have a significant portion of them having a 4-point improvement in their itch. So, that itch relief was very rapid.
There was similarly improved sleep. And so, the metrics for sleep mirrored the metrics of improvement for itch, and then also quality of life was very significantly improved. So, we have this metric for our quality of life index in dermatology, the DLQI [Dermatology Life Quality Index]. And over 50% of patients had a DLQI 0 to 1, which is no impact or minimal impact, and 90% of patients had a 4-point improvement. So, very significant improvement across metrics, itch, skin lesions, sleep disturbance, as well as quality of life.
Newsletter
Stay ahead of policy, cost, and value—subscribe to AJMC for expert insights at the intersection of clinical care and health economics.