
The opening plenary session addresses a key priority of ISPOR on the first full day of the annual conference.
Mary Caffrey is the Executive Editor for The American Journal of Managed Care® (AJMC®). She joined AJMC® in 2013 and is the primary staff editor for Evidence-Based Oncology, the multistakeholder publication that reaches 22,000+ oncology providers, policy makers and formulary decision makers. She is also part of the team that oversees speaker recruitment and panel preparations for AJMC®'s premier annual oncology meeting, Patient-Centered Oncology Care®. For more than a decade, Mary has covered ASCO, ASH, ACC and other leading scientific meetings for AJMC readers.
Mary has a BA in communications and philosophy from Loyola University New Orleans. You can connect with Mary on LinkedIn.

The opening plenary session addresses a key priority of ISPOR on the first full day of the annual conference.

Panelists at the leading health economics and outcomes conference discuss whether the FDA's process for allowing outside experts to weigh in on drug approvals needs changes.

Authors discuss the merits of using a pair of immunotherapy treatments aimed at separate targets.

About 5000 leaders in health economics and outcomes research will gather for the 2024 meeting, which has the theme, "HEOR: A Transformative Force for Whole Health.”

Investigators also examined which type of immunosuppression was associated with a higher likelihood of Kaposi sarcoma.

"Off the shelf" CAR T-cell therapies could offer a solution in chronic lymphocytic leukemia, where T-cell exhaustion creates treatment challenges.

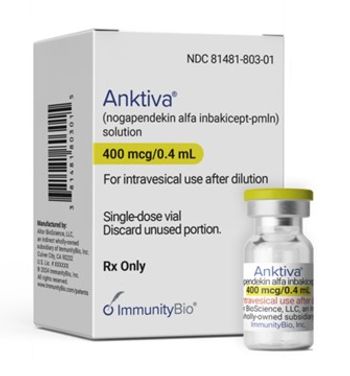
The principal investigator of the study leading to approval said this new immunotherapy could be a "game changer" in bladder cancer.
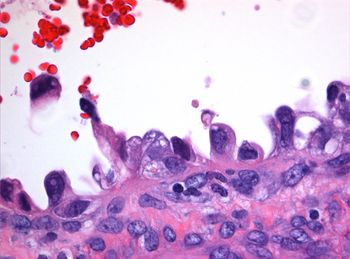
A tumor board looked to studies in ovarian cancer for guidance on use of immunotherapy in a rare case of clear cell carcinoma of the cervix.

News from Strategic Alliance Partners of The American Journal of Managed Care.




The annual legislative update at the Community Oncology Alliance Community Oncology Conference identified what Congress may focus on prior to the upcoming election.

FDA granted accelerated approval to trastuzumab deruxtecan-nxki for adult patients with unresectable or metastatic HER2-positive solid tumors who received prior systemic treatment and have no satisfactory alternative treatment options.

This panel at the Community Oncology Alliance conference discussed the impact of pharmacy benefit manager (PBM) response to the end of direct and indirect remuneration fees, which has been dramatic cuts to cancer drug reimbursement.

Jonathan E. Levitt, Esq, founding partner of boutique health care law firm Frier Levitt, LLC, discusses a recent class-action lawsuit brought against Johnson & Johnson in which an employee alleged a breach of fiduciary duty regarding her employer-sponsored pharmacy benefits.

After the end of the Oncology Care Model (OCM), practices are working with primary care providers, which has pros and cons.

A lawsuit that followed a health system revoking community oncologists' hospital privileges was the jumping off point for a discussion of how to manage this key relationship.

The authors say a standard method of assessing metabolic tumor volume would be needed for its use to become widespread.

The annual Community Oncology Conference comes on the heels of a cyberattack that has caused significant disruption to providers, and many have sought relief from payers in areas such as prior authorization.

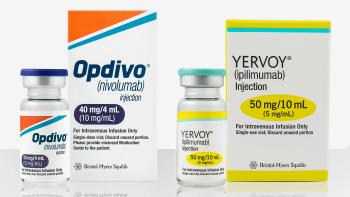
An alternative dose of ipilimumab and nivolumab had less toxicity than a conventional dose, the results show.

Results are consistent with the authors' prior findings on the possible effects of circadian rhythm on outcomes after immunotherapy.

The Princess of Wales is likely referencing adjuvant chemotherapy, but there is a growing field of immunoprevention, which seeks to target precancerous lesions or block heritable cancers.
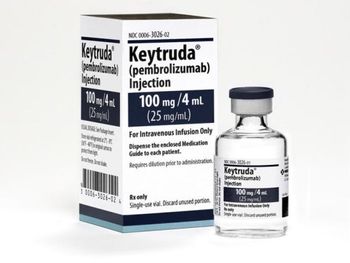
This is the second time the combination has failed to meet end points in a trial in non–small cell lung cancer (NSCLC).

The approval fills a void for patients with CLL or SLL whose disease progresses after treatment with a BTK inhibitor and a BCL-2 inhibitor; until now, there has been no standard of care.

As pressure from the Biden administration on UnitedHealth Group increases, physicians' groups weigh in following the unprecedented hack on Change Healthcare.

Results come amid the FDA's review of chimeric antigen receptor (CAR) T-cell therapy.

In his State of the Union Address, the president called for expanding the number of drugs subject to Medicare price negotiations and extending a $2000 out-of-pocket cap beyond seniors.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
